I'm not so familiar, sorry...
[Step 1] Molecules are ionized and crashes into fragments.
[Step2] Then, the ions are accelerated and induced into magnetic field.
[Step3] In the magnetic field, the ions are affected by Lorentz force F(N) and their obrit is bended.
F=q(vxxB) (q(C) the charge of ion: v(m"/"s): velocity of ion, xx: cross product, B(T): magnetic flux density).
And of course...
F=ma (m(kg): mass of ion, a(m"/"s^2): acceleration) and you can conclude:
a=(q(vxxB))/m
The point is that color(red)"orbit of lighter ions or fractions are bended"
color(red)"more easily than that of hevier ions."
Acceleration rate a is proportional to q/m.
[Step4] Sort ions or fractions by m/q(i.e. the reciprocal of q/m) and record the relative abundance of ions. This is how you can find a mass of the molecule or mass of fragments.
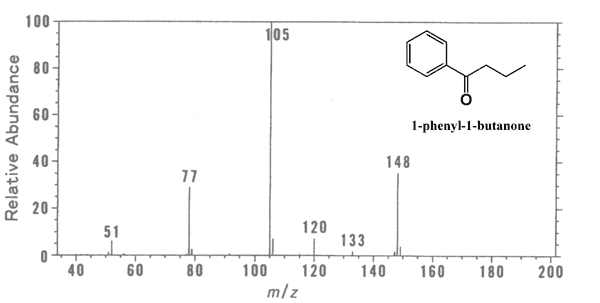
This is a mass spectrum for 1-phenyl-1-butanone(C_6H_5COC_3H_7). Cited from http://www2.odn.ne.jp/had26900/topics_&_items2/about_MS1.htm
What you can find from the result…
148: The mass of the whole molecule, and this is exactly the same as C_6H_5COC_3H_7.
77: The mass is same as the phenyl group, C_6H_5-. This implies that the molecule might have benzene ring.
105: 105-77=28 is the mass of carbonyl group(-CO-). Thus we can expect the molecule to have C_6H_5CO- group.


