What is meant by a stress on a reaction at equilibrium?
1 Answer
There are three 'Stress Factors' that affect the stability of an equilibrium:
1. Concentration Effects
2. Temperature Effects
3. Pressure-Volume Effects
Explanation:
Kinda long, but I had JPEGs in my files => Don't mind sharing.
A system (Reaction) at equilibrium is producing products as fast as it is producing reactants. This is known as the Law of Mass Action. That is ...
Rate of forward reaction = Rate of reverse reaction
When this condition exists, the reaction system is said to be in a 'stable' Dynamic Equilibrium'. While the concentrations of reactants and products appear to be constant, the chemical or physical process is still taking place.
To visualize this, think of the equilibrium process as being balanced on a See-Saw. Such as ...
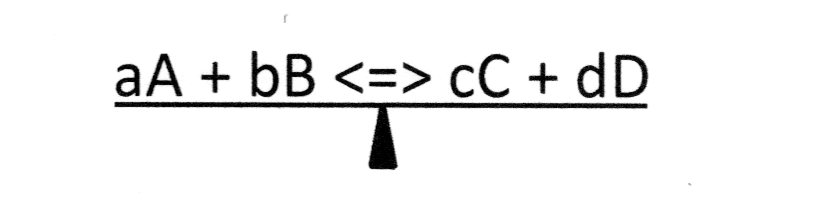
If a stress is applied, the balance will be disturbed and the See-Saw will tilt toward the side of the equation to which the stress factor is applied.
The reaction will then shift away from the applied stress and establish a 'new' equilibrium with different concentration values for the reactants and products. => LeChatelier's Principle
Consider Concentration Effects:
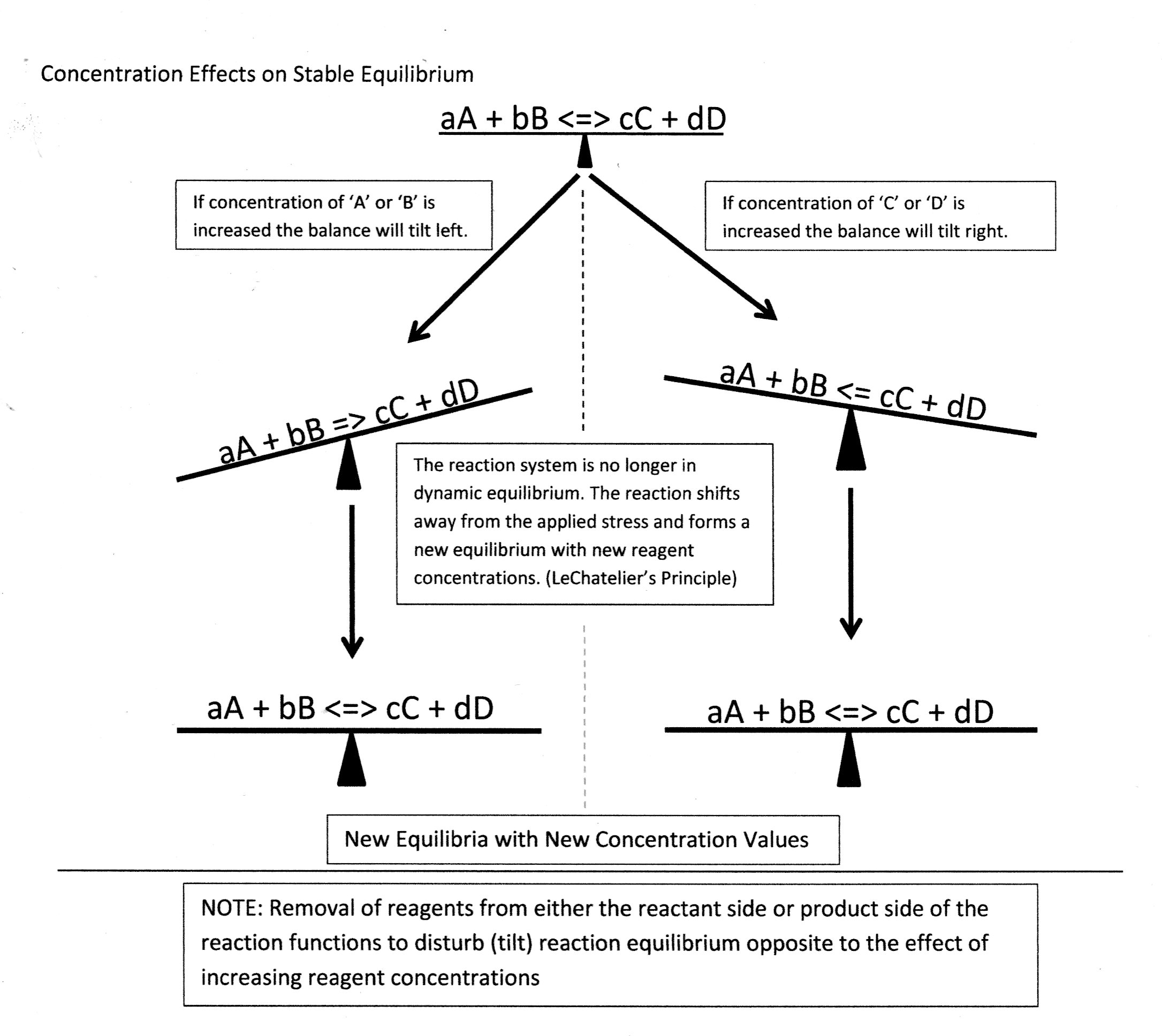
Concept Check:
Consider the Rxn:
Stress Factor => Removing
Consider the Rxn:
Stress Factor => Adding
Stress Factor => removing
Consider Temperature Effects:
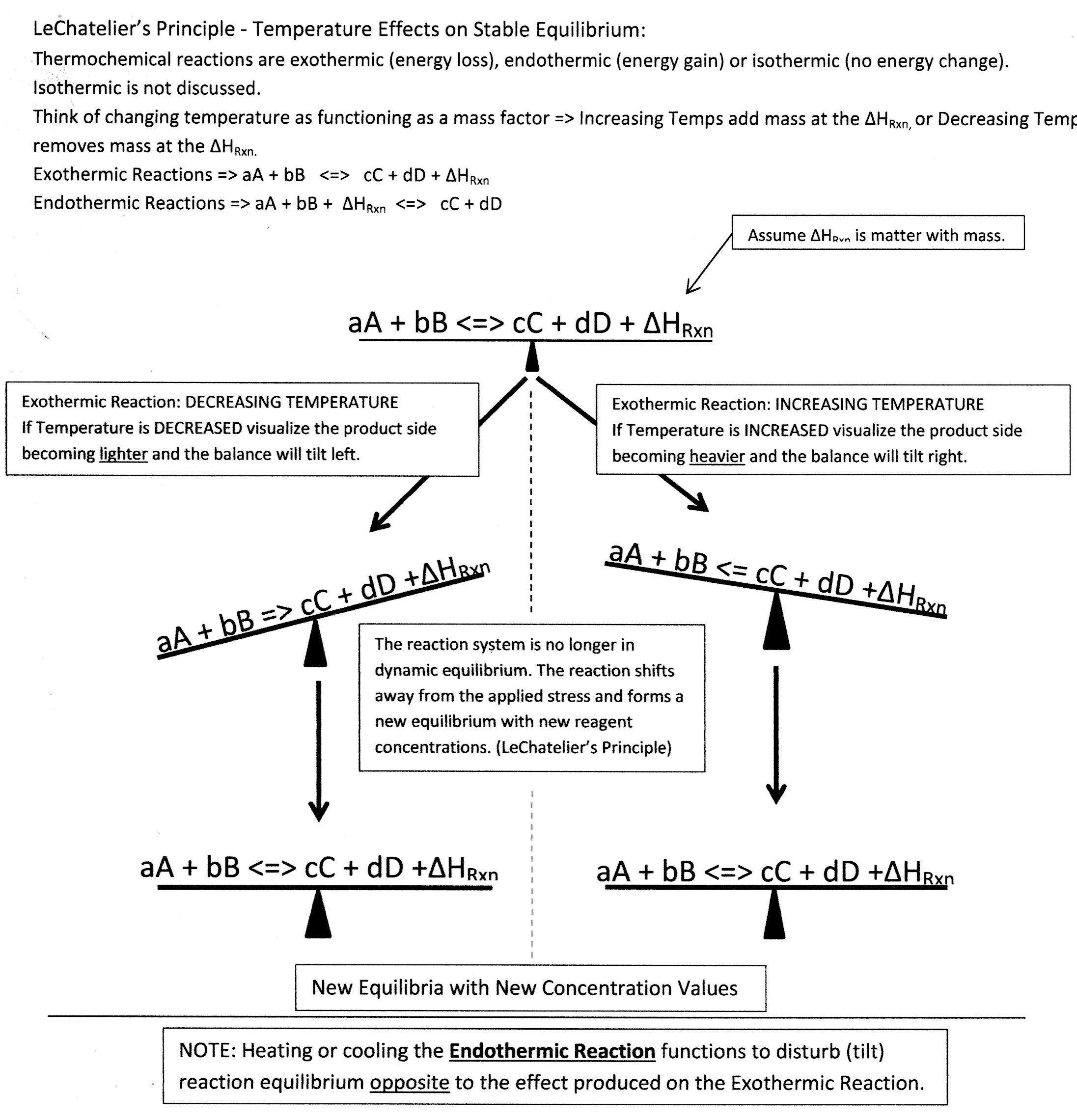
Concept Check:
Consider the reaction:
Stress Factor => Cooling rxn environment => product side is lighter => Rxn balance would tilt left => Rxn Shifts Right to establish new equilibrium.
Consider the reaction:
To increase decomposition => Stress Factor => Increase temperature around reaction environment => Rxn balance would tilt left => Rxn Shifts Right to establish new equilibrium.
Consider Pressure-Volume Effects:
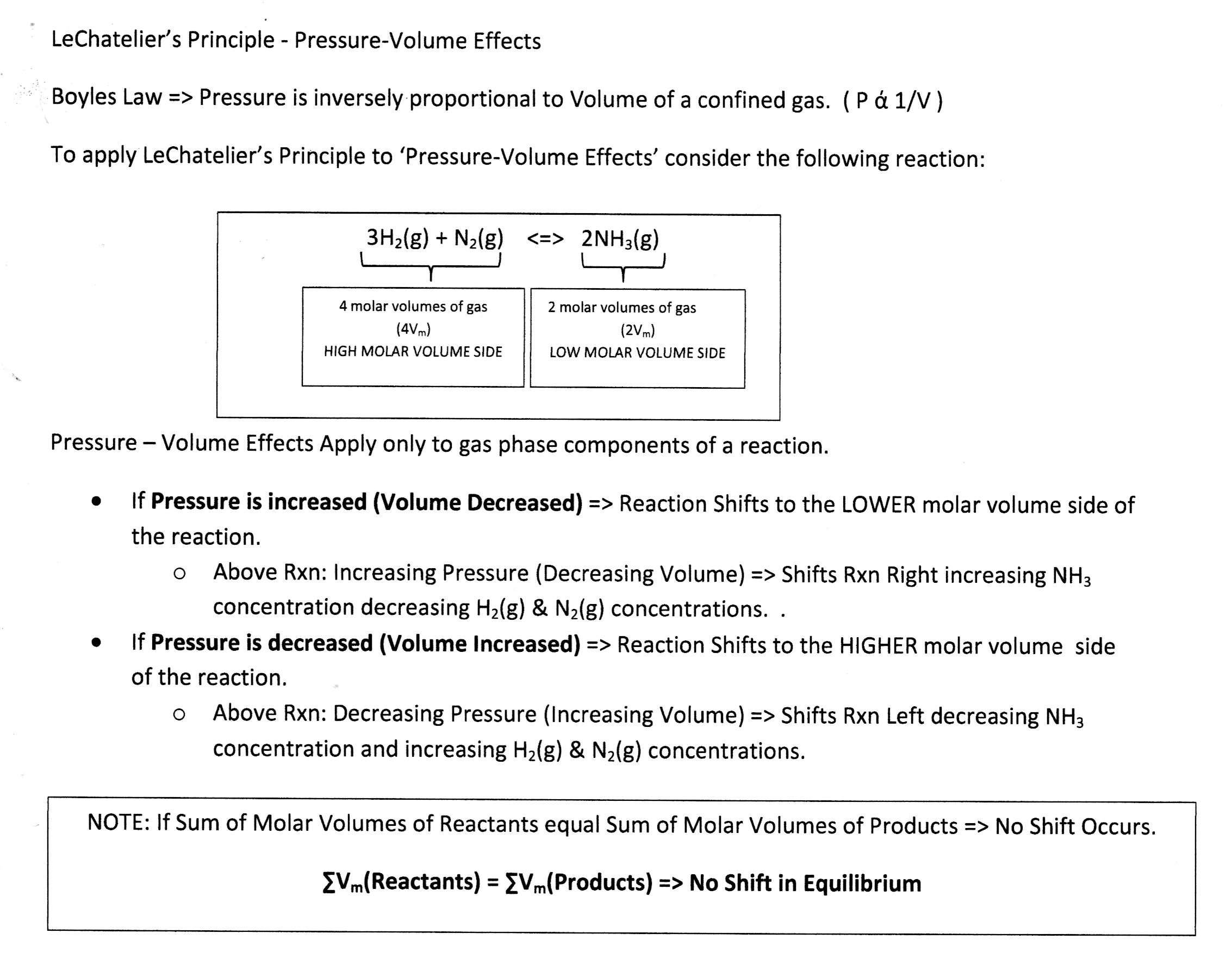
Concept Check: Increasing pressure will ...
Same Rxn but note => all gas phase components.

