What is nitrogen inversion?
1 Answer
Jan 4, 2018
It's a quantum tunneling effect where the nitrogen atom protons tunnel through a potential energy barrier that allows the molecule to invert its shape. Examples are
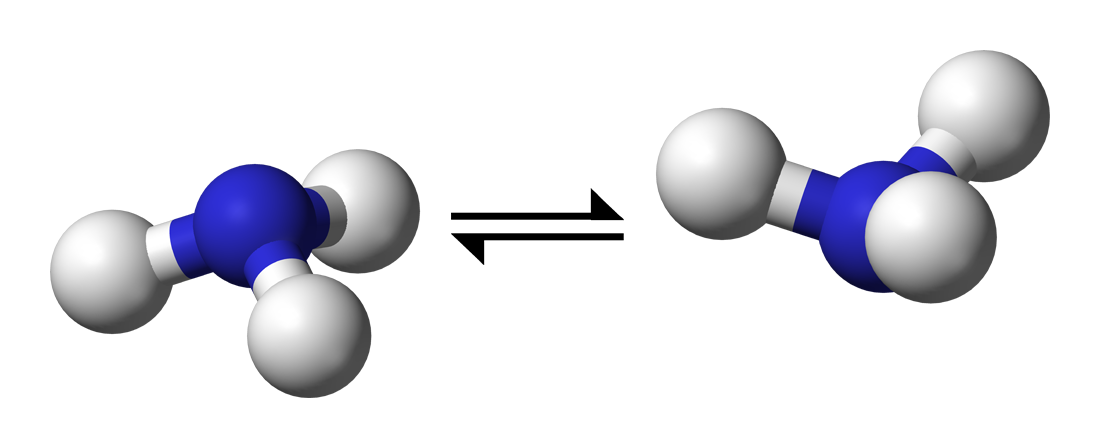
The potential energy double well can be represented as follows:
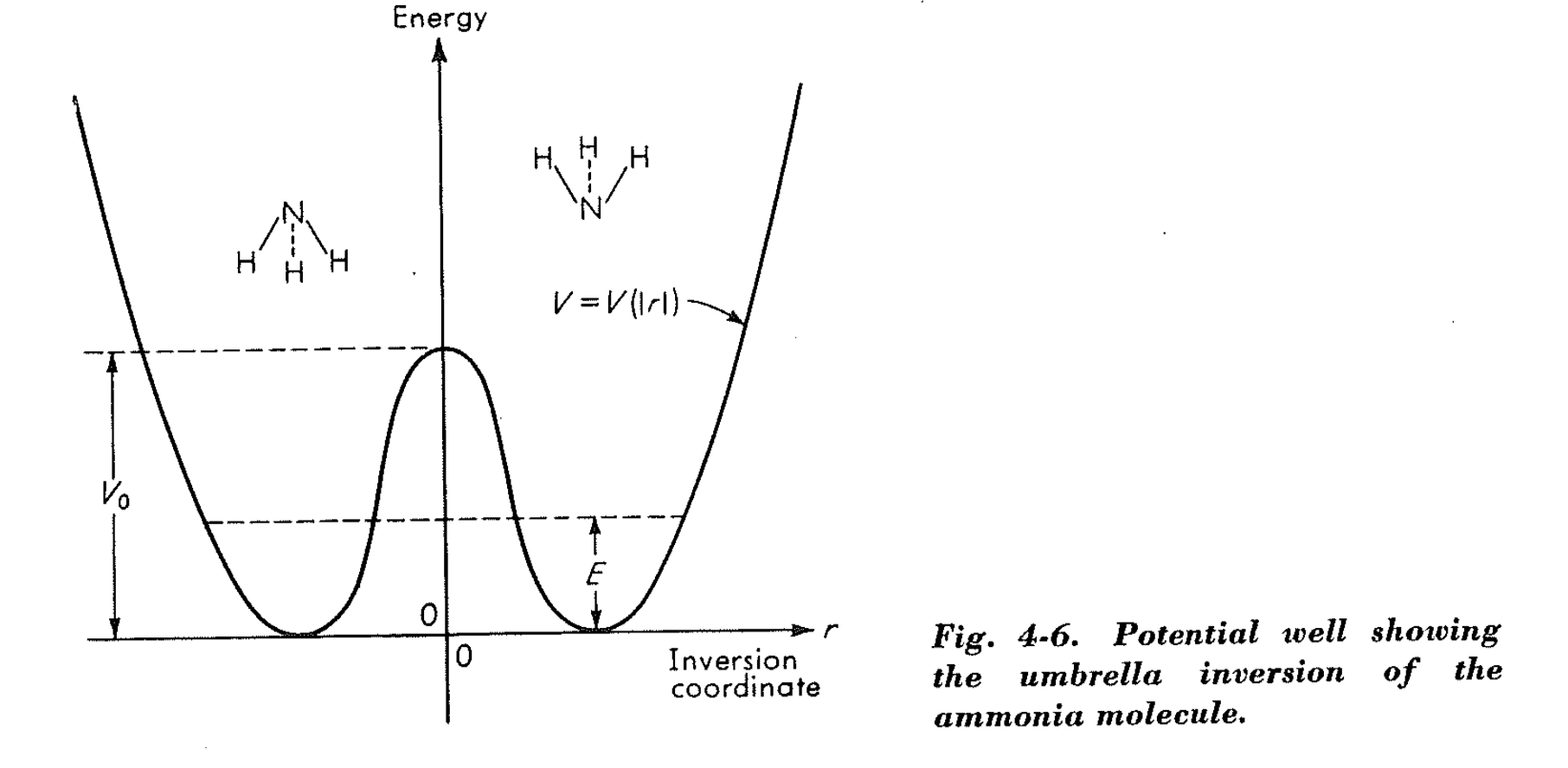
This activation energy is around
For comparison, a typical reaction activation energy is between

