What is relative number of atoms?
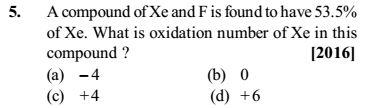
This is the question
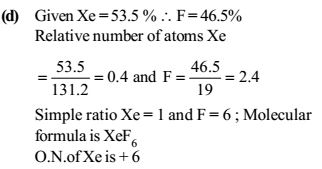
This is the answer given. Now, what is the 'relative number of atoms' that they have found out and why is it found out like this? I am not able to get the concept of 'relative number of atoms'.
This is the question
This is the answer given. Now, what is the 'relative number of atoms' that they have found out and why is it found out like this? I am not able to get the concept of 'relative number of atoms'.
1 Answer
The relative number of atoms is the empirical formula.
The relative number of atoms
A compound consists of two or more atoms joined together in a fixed ratio.
For example, in water,
In hydrogen peroxide,
We can't see the atoms, so all we can do is determine the relative numbers of each type.
For example, the molecular formula of hydrogen peroxide could be
The formula with the smallest relative numbers is the empirical formula.
Finding the empirical formula
The empirical formula is the simplest whole-number ratio of atoms (i.e., their relative numbers) in a compound.
The ratio of atoms is the same as the ratio of moles.
So, our job is to calculate the molar ratio of
Your compound contains 55.35 %
Assume that you have 100 g of sample.
Then it contains 53.5 g of
From this point on, I like to summarize the calculations in a table.
Although there are almost the same masses of
The empirical formula is

