What is selectivity? Is there any effect of selectivity in the halogenation reaction?
1 Answer
Selectivity is basically the tendency for a particular product to be made. It could be given in various subterminology:
- Regioselectivity - the tendency to form one constitutional isomer over another in a reaction.
This derives from the idea of adding certain atoms to different regions across the same bond. A common example is hydrohalogenation (
#"HX"# ) of asymmetrical alkenes, yielding the Markovnikov addition product.
- Stereoselectivity - the tendency to form one stereoisomer over another in a reaction.
An
#"S"_N2# reaction inverts the stereoconfiguration of a chiral compound, and thus is stereoselective for the opposite stereoisomer (EX:#"R config reactant"# #-># #"S config product"# ).
Halogenation of alkenes, i.e.
After the first
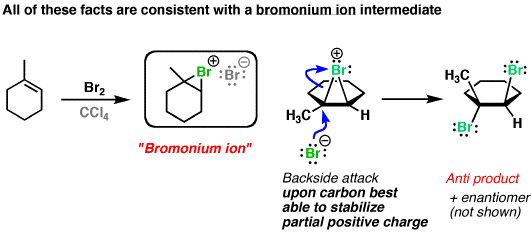
Having a backside-attack of a
You also don't get rearrangement after formation of the anti addition product, so no carbocation intermediate actually forms:
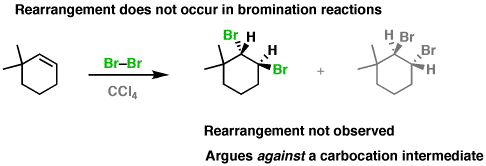
Therefore, we find that halogenation is definitely selective.

