What is the answer? With an explanation, please.
1 Answer
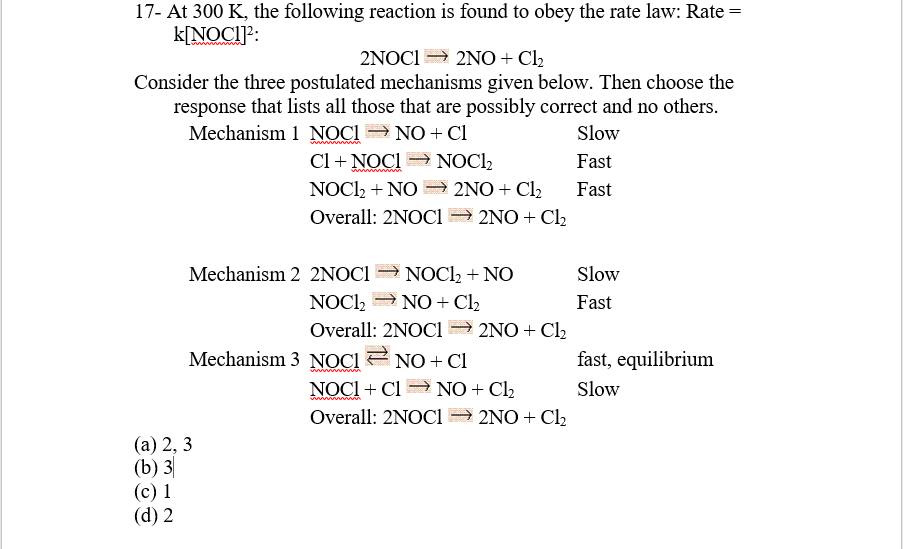
The essence of these problems is determining the rate determining step: which the rate law is derived from.
Consider,
In the first mechanism, the slow step is the RDS, and there is only one mole of
In the second mechanism, the slow step is the RDS, and there are two moles of
In the third mechanism, we'll need to do some analysis,
This isn't the RDS, but it makes the intermediate in the RDS (the slow step after). So let's substitute,
for the intermediate in the RDS and see what we come up with.
Consider,
where,
Hence, the only reliable mechanism we have that matches that rate law is

