What is the approximate energy of a photon having a frequency of #4*10^7# #Hz#?
1 Answer
Jul 26, 2016
Explanation:
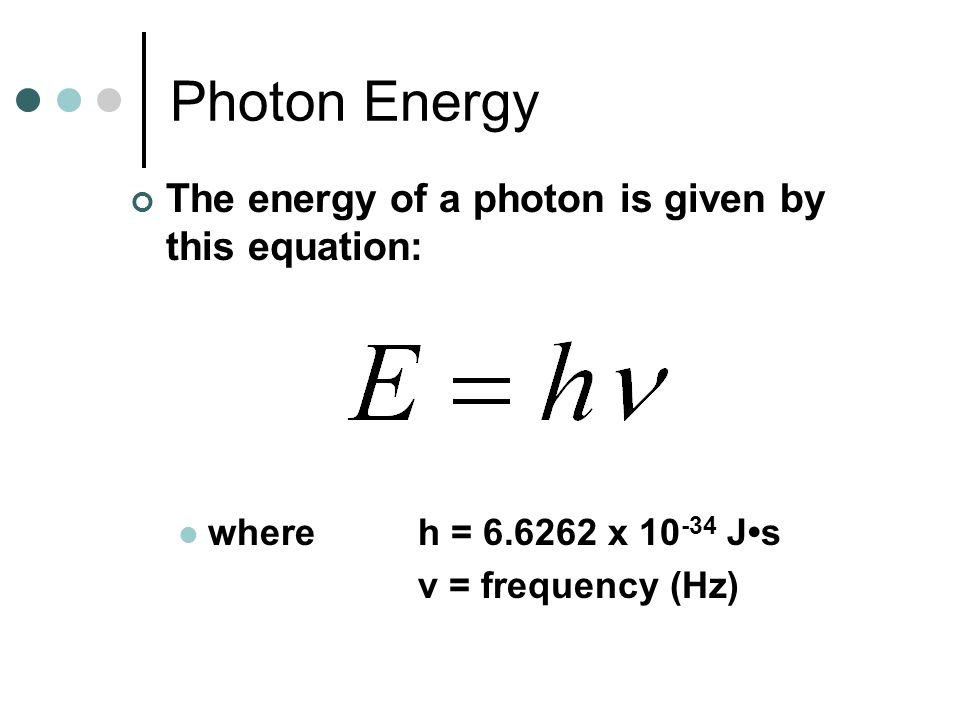
We know h and v, so all we have to do is plug the known values into the equation and solve for E like this:
Thus,
- I should also mention that the unit Hz is the same as
#s^(-1)# , I wrote it that way because I wanted you to see how the units canceled out.

