What is the bond polarity of CH4?
1 Answer
May 8, 2018
The dipole moment of a
Explanation:
We often say that a
However, the values are
The difference corresponds to 3% ionic character, so a
From experimental IR intensities of methane, we can infer that
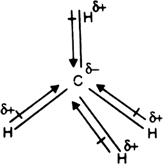
Theoretical calculations give varying results, but most experimental values are between 0.3 D and 0.4 D.

