What is the bond polarity of h2s?
1 Answer
The short version: Sulfur is more electronegative than hydrogen, so the
Explanation:
This leads to
However, the difference in polarity between
The longer version:
The difference in electronegativities of hydrogen (2.20) and sulfur(2.58) is almost exactly the same as that between hydrogen and carbon (2.55).
The
However, there is some polar character to a
Its dipole moment is only 0.3 D.
We should therefore expect an
Yet, the measured molecular dipole moment of
If this were due entirely to the polar
However, hydrogen sulfide has two lone pairs.
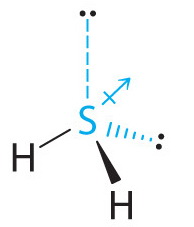
(from 2012 Book Archive)
Lone pairs contribute to the molecule's dipole moment even though they do not constitute a 'bond'.
The sulfur 'end' of the lone pair is positive, and the electron 'end' is negative, so one might think of a 'lone pair dipole' contributing to the polarity of the molecule in analogy to a bond dipole.
Thus, it may be the lone pairs that make the major contribution to the polarity of the molecule.

