What is the chemical equation for aluminum replacing hydrogen when the metal is placed in hydrochloric acid?
1 Answer
Explanation:
This is a single replacement (single displacement) reaction in which the aluminum replaces the hydrogen in hydrochloric acid, which produces aluminum chloride and hydrogen gas.
The general form for a single replacement reaction is:
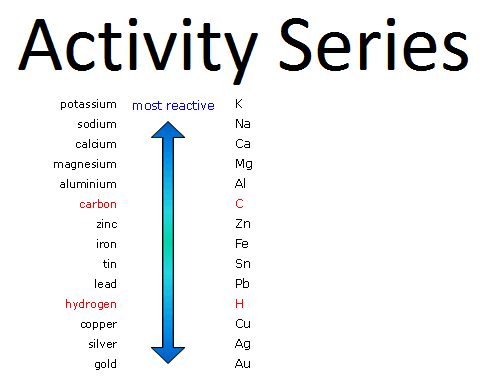
Not all possible single replacement reactions will occur. The element that replaces the element in the compound must be more reactive than the one it is replacing. We use an activity series to determine whether a single replacement reaction will occur.
You can see in the activity series below, aluminum is above hydrogen, so it is more reactive and will replace it in the reaction.