What is the chemical equation for titration of #HCl + NH_3#?
1 Answer
Explanation:
Hydrochloric acid,
#"NH"_ (3(aq)) + "HCl"_ ((aq)) -> "NH"_ 4"Cl"_ ((aq))#
In this particular reaction, the chloride anions,
#"NH"_ (3(aq)) + "H"_ ((aq))^(+) -> "NH"_ (4(aq))^(+)#
It's worth mentioning that because you're titrating a strong acid with a weak base, the pH of the resulting solution will be lower than
That is the case because this neutralization reaction produces the ammonium cation,
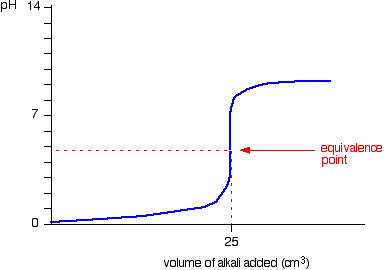
Assuming that you're running ammonia into hydrochloric acid, the titration curve will look like this