What is the combination reaction for #K(s) +Cl_2(g)#?
1 Answer
Explanation:
Potassium metal,
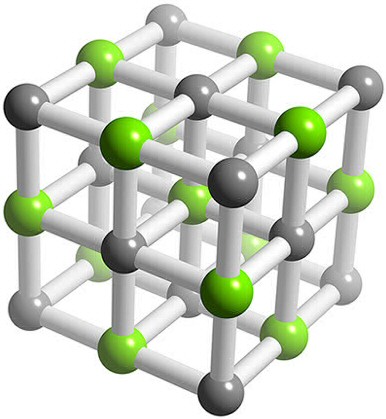
Potassium chloride's lattice structure looks like this
Here each potassium cation, shown here in dark gray, is surrounded by six chloride anions, shown here in green.
The balanced chemical equation that describes this synthesis reaction, also known as a combination reaction, looks like this
#2"K"_ ((s)) + "Cl"_ (2(g)) -> 2"KCl"_ ((s))#
You can also think about this reaction in terms of oxidation and reduction. Here potassium metal is being oxidized to potassium cations,
#2stackrel(color(blue)(0))"K"_ ((s)) + stackrel(color(blue)(0))"Cl"_ (2(g)) -> 2stackrel(color(blue)(+1))("K")stackrel(color(blue)(-1))("Cl")_ ((s))#