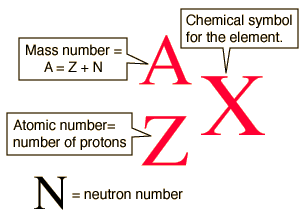What is the correct symbol for an isotope of iodine with 53 protons and 78 neutrons?
1 Answer
Dec 31, 2015
Explanation:
The atomic number is the number of protons in the nuclei of the element's atoms, is designated Z, and is written as a subscript in front of the element's symbol. The mass number, A, is the sum of the protons, Z, and neutrons, N, and is written as a superscript in front of the element's symbol.