What is the difference between 3-methylcyclohexene and 4-methylcyclohexene on the basis of mass spectrosvopy?
2 Answers
Consider each, respectively,

The fragmentation of each will be different, because they have different constitutions. To be sure, their fragment peaks will have slightly different
I can think of two significant differences in the fragmentation patterns.
Explanation:
1. Loss of the sidechain
Both compounds can lose the methyl group to form a cyclohexenyl cation with mass M - 15 = 81.
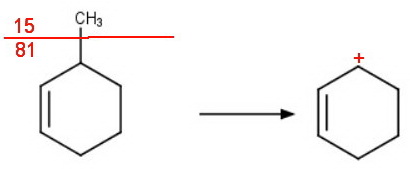
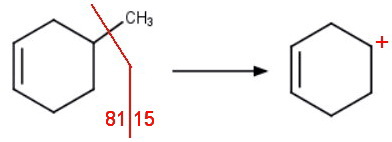
The peak at mass 81 will probably be the parent peak for both hydrocarbons.
However, the fragment from the 3-methyl isomer is a stabilized allylic carbocation. It should be perhaps twice as intense as for the other isomer.
Since both peaks are normalized to 100 %, the only difference we would see is that the other peaks would appear to be only half as intense as those in the 4-methyl isomer.
2. The retro-Diels-Alder reaction
Cyclohexenes can undergo a retro-Diels-Alder reaction.
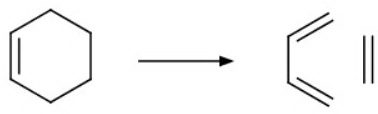
A retro-Diels-Alder reaction with our 3-methyl and 4-methyl compounds gives different fragments.
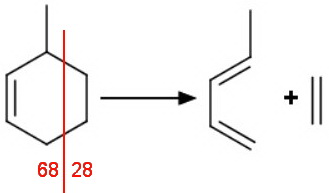
The second-most intense peak will probably be at 68, corresponding to loss of ethylene (M - 28).
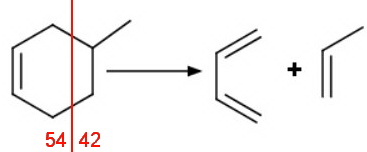
The second-most intense peak will probably be at 54, corresponding to loss of propylene (M - 42).


