What is the difference between a sigma bond and a pi bond?
1 Answer
A σ bond has cylindrical symmetry; a π bond has a nodal plane that includes the bond axis.
Explanation:
A σ bond has cylindrical symmetry; a π bond has a nodal plane that includes the bond axis.
A σ bond comes from the end-to-end overlap of the bonding orbitals. The common forms of sigma bonds are shown below.
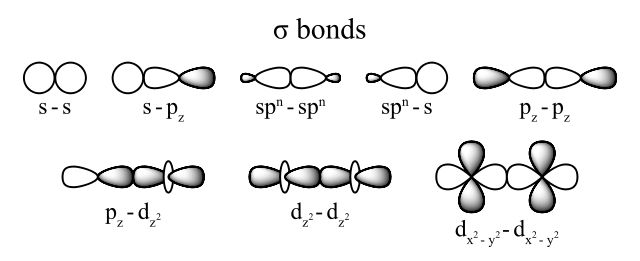
Sigma bonds are cylindrically symmetrical. This means that if you draw a line along the internuclear axis, then you can rotate the bond any number of degrees. If you look only at only that bond, you cannot see a difference after rotation.
A π bond comes from the side-to-side overlap of
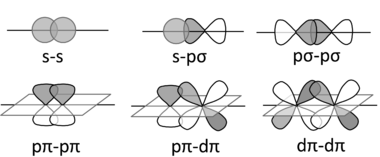
π bonds have a nodal plane that is perpendicular to the lobes of the

