What is the difference between oxidation number and oxidation state?
1 Answer
Oxidation number might be easier to understand at the high school level, but oxidation state and oxidation number are synonymous in many cases.
The only time I can think of where they are different are in coordination complexes.
In
On the other hand,
OXIDATION STATE EXAMPLE
The oxidation state is the hypothetical charge if the bond that the atom makes with a second atom is 100% ionic in character, and if after accounting for electronegativities, the more electronegative atom in the pair has the more negative oxidation state.
It isn't ever exactly the case for a real bond to have 100% ionic character (i.e. to transfer 100% of the electron density into another atom's valence orbitals), but oxidation states turn out to be a good charge accounting scheme.
Consider the following
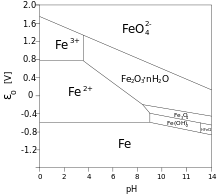
For example, if we are at
#\mathbf(stackrel(color(blue)(+3))("Fe"^(3+))(aq) + 4stackrel(color(blue)(+1))("H")_2stackrel(color(blue)(-2))("O")(l)-> stackrel(color(blue)(+6))("Fe")stackrel(color(blue)(-2))("O"_4^(2-))(aq) + 8stackrel(color(blue)(+1))("H"^(+))(aq) + 3e^(-))#
#E_"ox"^@ > "0.8 V"# (We started in the
#"Fe"^(3+)# region on the upper left side of the graph, and we moved upwards to oxidize.)
For this half-reaction, we've oxidized iron(III)
So, iron attains a
OXIDATION NUMBER VS. STATE EXAMPLE
One difference I can find is in
The oxidation number of titanium here is written as
This compound has a

