For #"sulfur trioxide"# we must distribute #3xx6=18*"valence electrons"#. While #S(=O)_3# is a possible representation, most inorganic chemists would write the Lewis structure with the assignment of formal charge....#O=stackrel(+2)S(-O^(-))_2#... #/_O-S-O=120^@#.
On the other hand for sulfurous acid, #H_2SO_3#, we have# 4xx6+2=13*"valence electron pairs"# to distribute..
And thus #O=stackrel(ddot)S(-OH)_2#...the central sulfur is #sp_3-"hybridized"#, and the electron pairs assume a tetrahedral geometry. But molecular geometry is described in terms of ATOMS not electron pairs...and so the geometry around sulfur is pyramidal...#/_O-S-O=105-6^@#.,,,due to the influence of the sulfur lone pair.
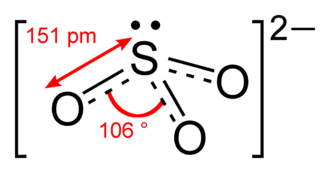
I take it you can assign the number of valence electrons in each molecule correctly... Each sulfur, and each oxygen has 6 valence electrons to distribute somehow, and we add an extra 2 electrons to account for the negative charge.


