What is the dissociation equation of Al2Cl6(s)?
1 Answer
Mar 13, 2018
Explanation:
Even at the sublimation temperature (180 °C), it exists as the dimer.
At higher temperatures, it dissociates into the monomer.
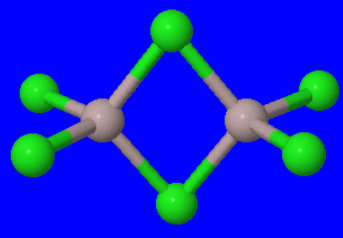
#"Al"_2"Cl"_6 ⇌ 2"AlCl"_3#
In the dimer, bridging
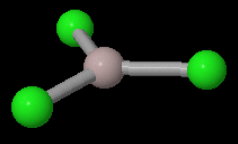
The monomer has a trigonal planar structure.