What is the E-Z designation of the following compound?
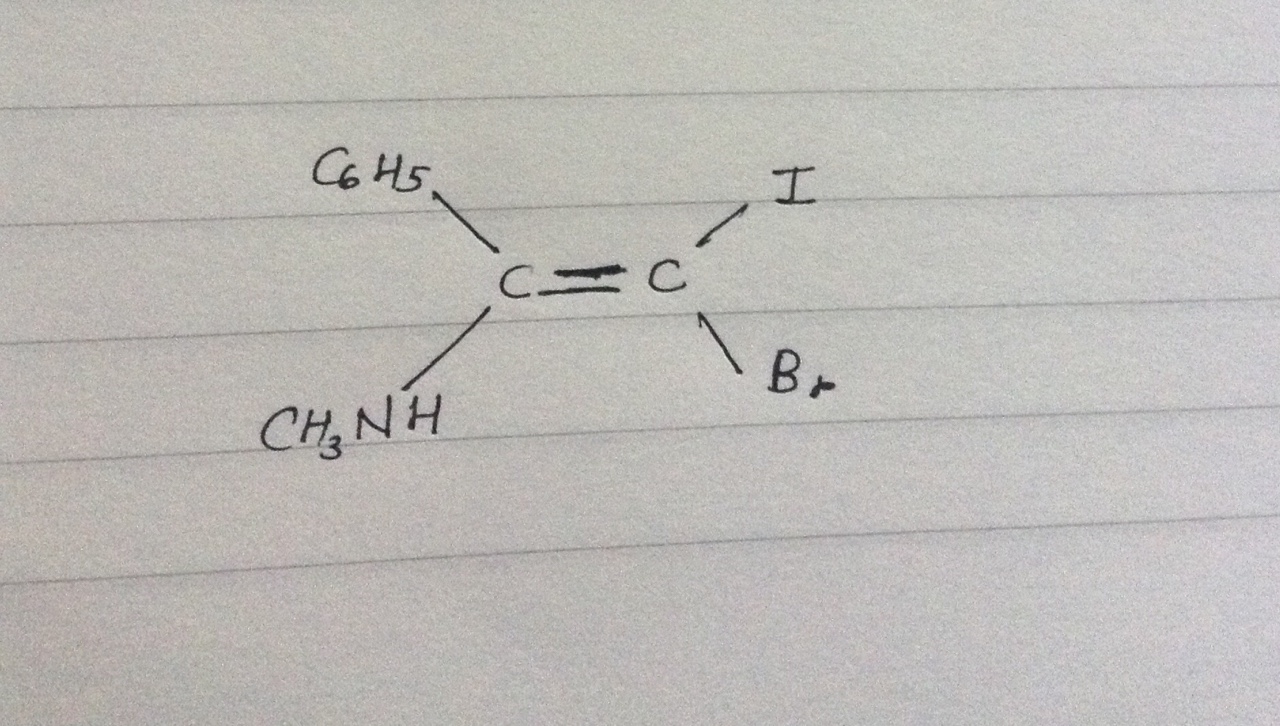
1 Answer
Dec 5, 2017
That is based on the Cahn-Ingold-Prelog (CIP) nomenclature priorities.
And we would get the
You would consider:
- Atomic number of the first atoms around the stereocenter
- Going one atom out if there is a tie
- Treating multiple bonds as "ghost atoms" in further single-bond branching
In this case, all of these first atoms around the stereocenter are different atomic number. So we only need to consider rule 1 to get:
- Priority 4 =
#"C"_6"H"_5# - Priority 3 =
#"CH"_3"NH"# - Priority 2 =
#"Br"# - Priority 1 =
#"I"#
Then, you divide the molecule in two, bisecting the double bond. This gives a higher priority on
Since

