What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
1 Answer
Jul 12, 2014
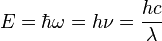
1 mol photons =
Energy of one photon (
Energy of one mole photon (
Energy of one mole photon (

