What is the energy, in joules, of a wave with a frequency of #6.78 * 10^7# Hz? What type of wave is this?
1 Answer
Dec 11, 2015
I think it is a FM radio signal with photon energy of around
Explanation:
Let us check (as an exercise) the wavelength
where:
so you get:
considering:
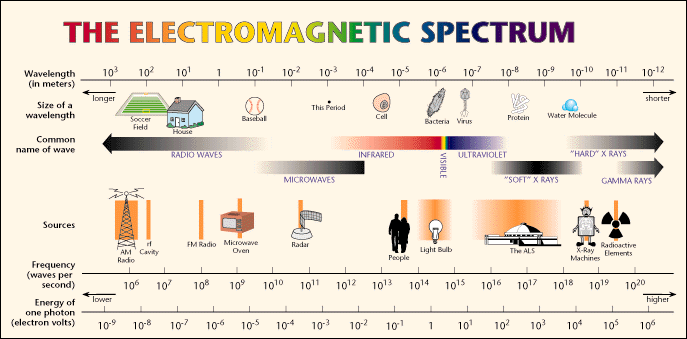
I would say that it falls into the FM radio range.
The energy can be calculated from Einstein's Relationship:
Where:
So:

