What is the energy profile in *cis*- and *trans*-1-bromo-4-methylcyclohexane?
1 Answer
The structure of 1-bromo-4-methylcyclohexane is
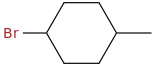
C-1 and C-4 are not chiral centres, so there are no optical isomers. The molecule, however, exists as a pair of cis and trans isomers.
cis-1-bromo-4-methylcyclohexane
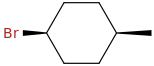
and
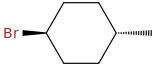
trans-1-bromo-4-methylcyclohexane
In a cis-1,4-disubstutited cyclohexane, one substituent is always equatorial, and the other is always axial.
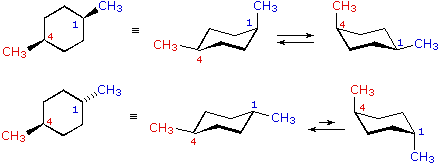
In the trans isomer, both substituents are either both equatorial or both axial.
The 1,3-diaxial CH₃ interactions amount to 7.6 kJ/mol. The 1,3-diaxial Br interactions amount to 2.8 kJ/mol.
trans-1,4-Dibromocyclohexane can exist in the diequatorial form with no 1,3-diaxial interactions. That is the most stable conformer.
The diaxial conformer would have (7.6 + 2.8) kJ/mol = 10.4 kJ/mol of steric strain. This is the least stable conformer
In cis-1-bromo-4-methylcyclohexane, the conformer with an axial CH₃ has 7.6 kJ/mol of strain. The conformer with an axial Br has 2.8 kJ/mol of strain.
The major conformer will have the CH₃ group equatorial.

