What is the equation that connects pH and its effect on electric potential of an electrochemical cell?
1 Answer
Well, first off, you'll need to begin with the Nernst equation:
E_(cell) = E_(cell)^@ - (RT)/(nF) lnQEcell=E∘cell−RTnFlnQ where:
E_(cell)Ecell is the overall cell potential.""^@∘ indicates"1 atm"1 atm and25^@ "C"25∘C .RR andTT are known from the ideal gas law.nn is the mols of electrons reportedly transferred in the redox reaction.F = "96485 C/mol e"^(-)F=96485 C/mol e− is Faraday's constant.
Note that
FROM THE PERSPECTIVE OF POURBAIX DIAGRAMS
The
This can be seen in Pourbaix diagrams:
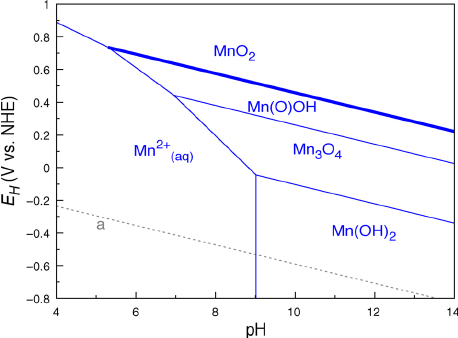
These are
- Diagonal lines indicate an
E_(cell)^@E∘cell that is"pH"pH -dependent. - Vertical lines indicate
100%100% "pH"pH dependence. - Horizontal lines indicate
0%0% "pH"pH dependence.
As a cursory example, for a given
"Mn"_3"O"_4(s) + 4"H"_2"O"(l) + 2e^(-) rightleftharpoons 3"Mn"("OH")_2(s) + 2"OH"^(-)(aq)Mn3O4(s)+4H2O(l)+2e−⇌3Mn(OH)2(s)+2OH−(aq)
It shifts towards
FROM THE PERSPECTIVE OF THE NERNST EQUATION
Or, using the Nernst equation, we can investigate the
Q = \frac(prod_i^(N_P) [P_i]^(nu_i))(prod_j^(N_R) [R_j]^(nu_j))Q=∏NPi[Pi]νi∏NRj[Rj]νj ,where:
prod_k^NN∏k indicates a multiplication of terms1, 2, . . . , k, . . . , N1,2,...,k,...,N .ii is the index of individual product concentrations[P_i][Pi] being multiplied together.jj is the index of individual reactant concentrations[R_j][Rj] being multiplied together.nu_kνk is the stoichiometric coefficient of thekk th reactant or product. Note that in general,i ne ji≠j , meaning the number of products and reactants may differ.
So, we have the following conditions:
As the
"pH"pH decreases, the solution is more acidic, so10^(-"pH") = ["H"^(+)]10−pH=[H+] increases and10^("pH" - 14) = ["OH"^(-)]10pH−14=[OH−] decreases.
- If
"H"^(+)H+ is a product,QQ therefore increases, and the nonstandard cell potential decreases. - If
"H"^(+)H+ is a reactant,QQ therefore decreases, and the nonstandard cell potential increases. - If
"OH"^(-)OH− is a product,QQ therefore decreases, and the nonstandard cell potential increases. - If
"OH"^(-)OH− is a reactant,QQ therefore increases, and the nonstandard cell potential decreases.
This is very situational, so it's not exactly possible to write one single equation for this, unless we make some assumptions.
PLAUSIBLE EQUATION
If the reaction does have an
E_(cell) = E_(cell)^@ - (RT)/(nF) ln (Q^"*"cdot["H"^(+)]^(pmnu_("H"^(+))))Ecell=E∘cell−RTnFln(Q*⋅[H+]±νH+) where:
Q^"*"Q* is the"pH"pH -independent reaction quotient with["H"^(+)][H+] separated out.nu_("H"^(+))νH+ is the stoichiometric coefficient of"H"^(+)H+ in the reaction.- In the
pm± fornu_("H"^(+))νH+ , the(+)(+) indicates"H"^(+)H+ product, while the(-)(−) indicates"H"^(+)H+ reactant.
We could write
E_(cell) = E_(cell)^@ - (RT)/(nF) ln Q^"*" - (RT)/(nF) ln (["H"^(+)]^(pmnu_("H"^(+))))Ecell=E∘cell−RTnFlnQ*−RTnFln([H+]±νH+)
= E_(cell)^@ - (RT)/(nF) ln Q^"*" - (pmnu_("H"^(+))RT)/(nF) ln (10^(-"pH"))=E∘cell−RTnFlnQ*−±νH+RTnFln(10−pH)
= E_(cell)^@ - (RT)/(nF) ln Q^"*" + "pH" cdot (pmnu_("H"^(+))RT)/(nF) ln 10=E∘cell−RTnFlnQ*+pH⋅±νH+RTnFln10
Rearrange this further to get:
E_(cell) - E_(cell)^@ + (RT)/(nF) ln Q^"*" = "pH" cdot (pmnu_("H"^(+))RT)/(nF) ln 10Ecell−E∘cell+RTnFlnQ*=pH⋅±νH+RTnFln10
"pH" = (E_(cell) - E_(cell)^@ + (RT)/(nF) ln Q^"*")/((pmnu_("H"^(+))RT)/(nF) ln 10)pH=Ecell−E∘cell+RTnFlnQ*±νH+RTnFln10
= ((nF)/(RT)(E_(cell) - E_(cell)^@) + ln Q^"*")/(pmnu_("H"^(+))ln 10)=nFRT(Ecell−E∘cell)+lnQ*±νH+ln10
Since
color(blue)(barul|stackrel(" ")(" ""pH" ~~ ((nF)/(2.303RT)(E_(cell) - E_(cell)^@) + log Q^"*")/(pmnu_("H"^(+)))" ")|) keeping in mind that
Q^"*" neglects the["H"^(+)] that would have been in the mass action expression, and that whether it was a product or reactant changes the sign ofnu_("H"^(+)) .

