What is the flux used in the extraction of cast iron from hamatite ore?
1 Answer
Feb 21, 2018
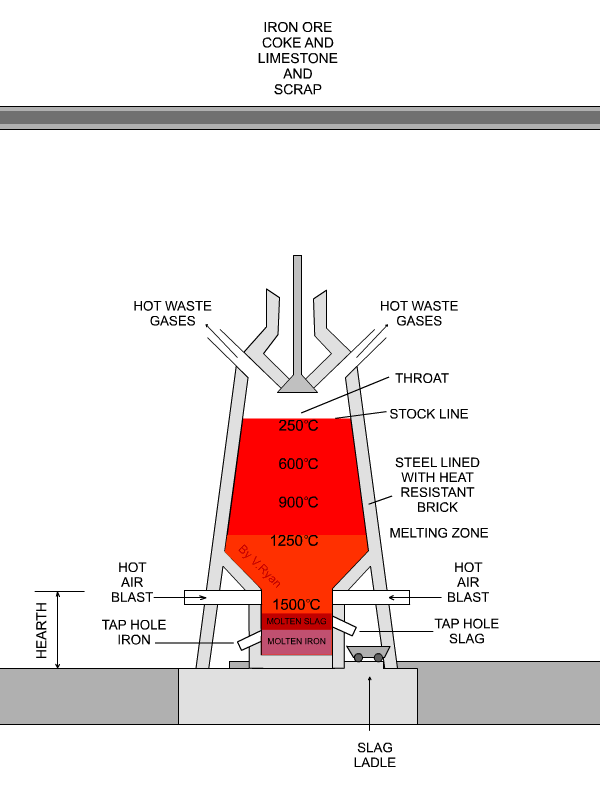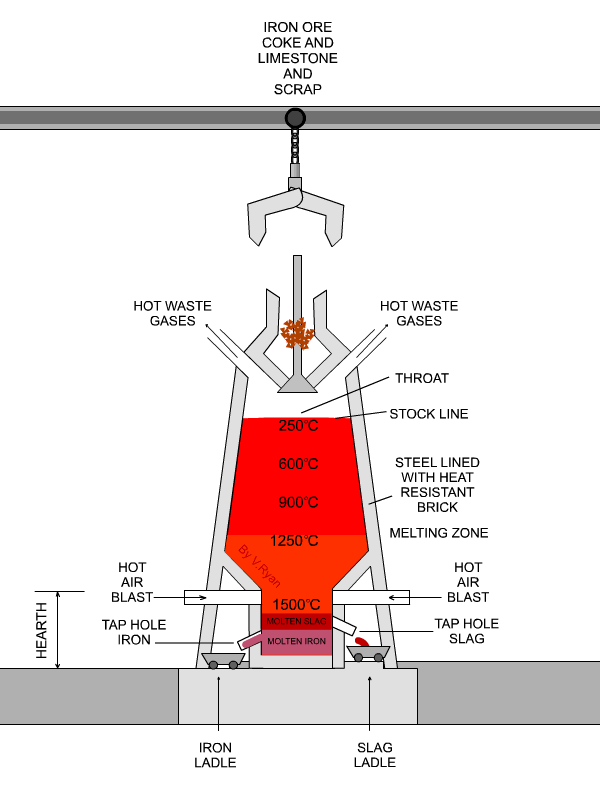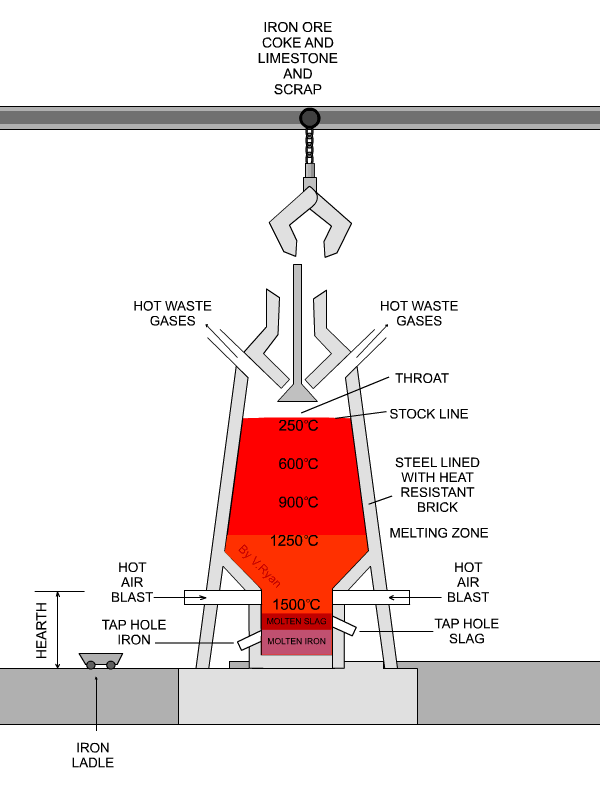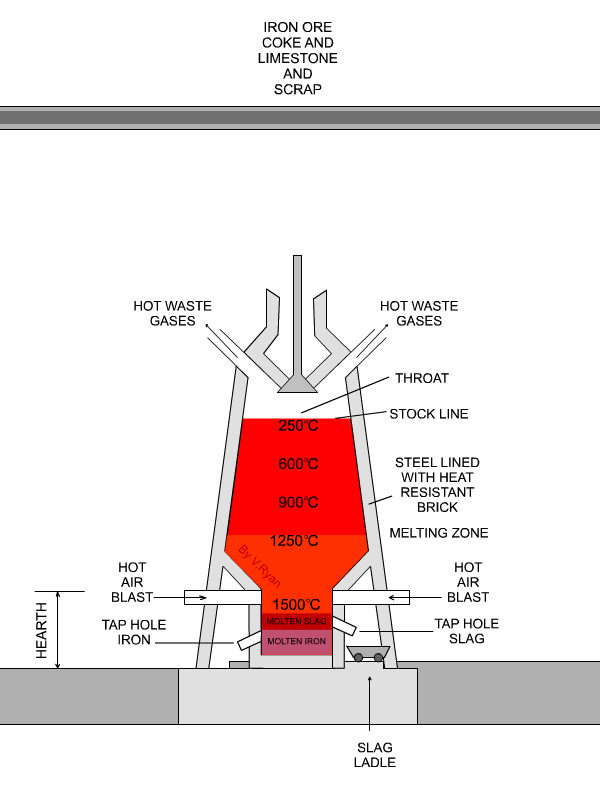
The flux is limestone (calcium carbonate).
Explanation:
The limestone decomposes at about 1000 °C to form
"CaCO"_3 → "CaO + CO"_2CaCO3→CaO + CO2
underbrace("CaO")_color(red)("basic oxide") + underbrace("SiO"_2)_color(red)("acidic oxide") → underbrace("CaSiO"_3)_color(red)("a salt")
The slag melts and floats over the denser molten iron, and openings in the side of the furnace allow them to run off separately.
 www.technologystudent.com
www.technologystudent.com

