What is the formula for copper (ll) phosphate?
1 Answer
Explanation:
The first thing to note here is that the name of the compound provides you with the charge of the cation.
Copper,
These Roman numerals describe the oxidation state of the transition metal in a given compound. In this case, the Roman numeral (II) means that copper has a
You can thus say that the cation will be
#"Cu"^(2+) -># the copper(II) cation
Now for the anion. Phosphate is the name given to a polyatomic ion that contains
- one atom of phosphorus,
# 1 xx "P"# - four atoms of oxygen,
#4 xx "O"#
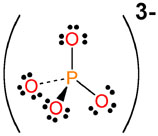
As you can see, the phosphate anion carries a
#"PO"_4^(3-) -># the phosphate anion
Now, ionic compounds must be electrically neutral, i.e. the overall positive charge coming from the cations must be balanced by the overall negative charge coming from the anions.
In this case, you have
#"Ca"^(2+)" "# and#" ""PO"_4^(3-)#
You will need
#color(blue)(3) xx ["Cu"^(color(red)(2+))] + color(red)(2) xx ["PO"_ 4^(color(blue)(3-))] -> "Cu"_ 3("PO"_ 4 )_ 2#
Therefore, you can say that you have
#"Cu"_3("PO"_4)_2 -># copper(II) phosphate

