What is the formula of dinitrogen pentoxide?
1 Answer
Jun 4, 2017
Explanation:
Lets have a look at the words involved:
#di# nitrogen and#pent# oxide.
The
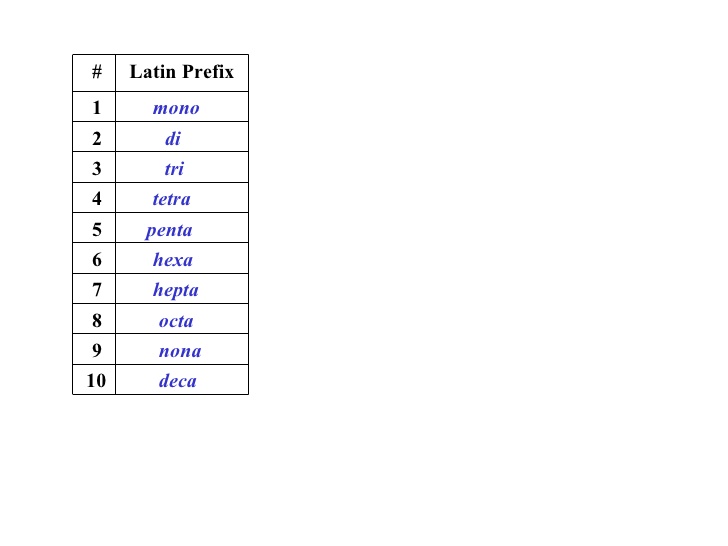
Using this knowledge (note that "penta" has the "a" removed for words starting with a vowel like "oxide"), it is possible to say that there are 2 nitrogen's and 5 oxygen's in this chemical equation, thus
Hope that Helped!
https://www.slideshare.net/mlacoursiere/c20-review-unit-01-matter-energy-and-the-periodic-table


