What is the free energy for the dissolution of solid sodium chloride in water at 25C?
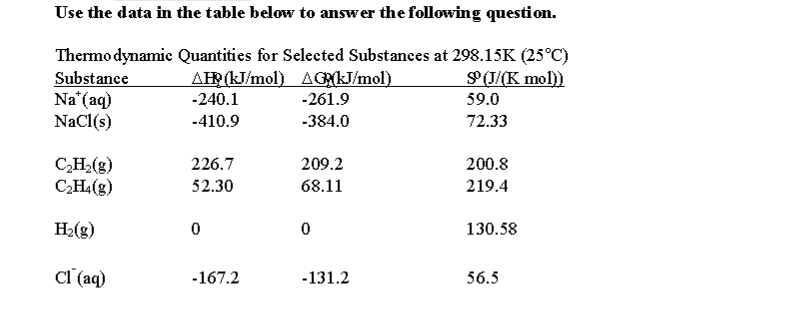
A) What is the free energy for the dissolution of solid sodium
chloride in water at 25C?
NaCl(s) <-> Na+(aq) + Cl-(aq)
B) What is the solubility product constant for sodium chloride
in water at 25C?

A) What is the free energy for the dissolution of solid sodium
chloride in water at 25C?
NaCl(s) <-> Na+(aq) + Cl-(aq)
B) What is the solubility product constant for sodium chloride
in water at 25C?
1 Answer
A) Given the reaction:
#"NaCl"(s) stackrel("H"_2"O"(l)" ")(->) "Na"^(+)(aq) + "Cl"^(-)(aq)#
The
#color(blue)(DeltaG_"rxn"^@) = sum_P nu_PDeltaG_(f,P)^@ - sum_R nu_RDeltaG_(f,R)^@#
#= [nu_("Na"^(+)(aq))DeltaG_(f,Na^(+)(aq))^@ + nu_("Cl"^(-)(aq))DeltaG_(f,Cl^(-)(aq))^@] - [nu_("NaCl"(s))DeltaG_(f,NaCl(s))^@]#
#= [(1)(-"261.9 kJ/mol") + (1)(-"131.2 kJ/mol")] - [(1)(-"384.0 kJ/mol")]#
#= -"261.9 kJ/mol" - "131.2 kJ/mol" + "384.0 kJ/mol"#
#= color(blue)(-"9.1 kJ/mol")#
It should be this small; that's fine, for dissolving solutes.
B)
The larger the
Since we have
#cancel(DeltaG_"rxn")^(0) = DeltaG_"rxn"^@ + RTlncancel(Q)^(K_"sp")#
#=> DeltaG_"rxn"^@ = -RTlnK_"sp"#
#=> color(blue)(K_"sp") = e^(-DeltaG_"rxn"^@"/"RT)#
#= e^(-(-"9.1 kJ/mol")"/"[("0.008314472 kJ/mol"cdot"K")("298.15 K")]#
#= color(blue)(39.29)#
And indeed,

