What is the frequency of a wave carrying #8.35 * 10^-18# J of energy?
1 Answer
Explanation:
The relationship between the energy carried by a wave and its frequency is given by the Planck - Einstein equation
#color(blue)(E = h * nu)" "# , where
This tells you that the energy of the wave is proportional to its frequency, i.e. higher frequency waves will carry more energy than lower frequency ones, with Planck's constant being the factor of proportionality.
So, plug in your values and solve for
#E = h * nu implies nu = E/h#
#nu = (8.35 * 10^(-18)color(red)(cancel(color(black)("J"))))/(6.626 * 10^(-34)color(red)(cancel(color(black)("J"))) * "s") = 1.26 * 10^16"s"^(-1) = color(green)(1.26 * 10^16"Hz"#
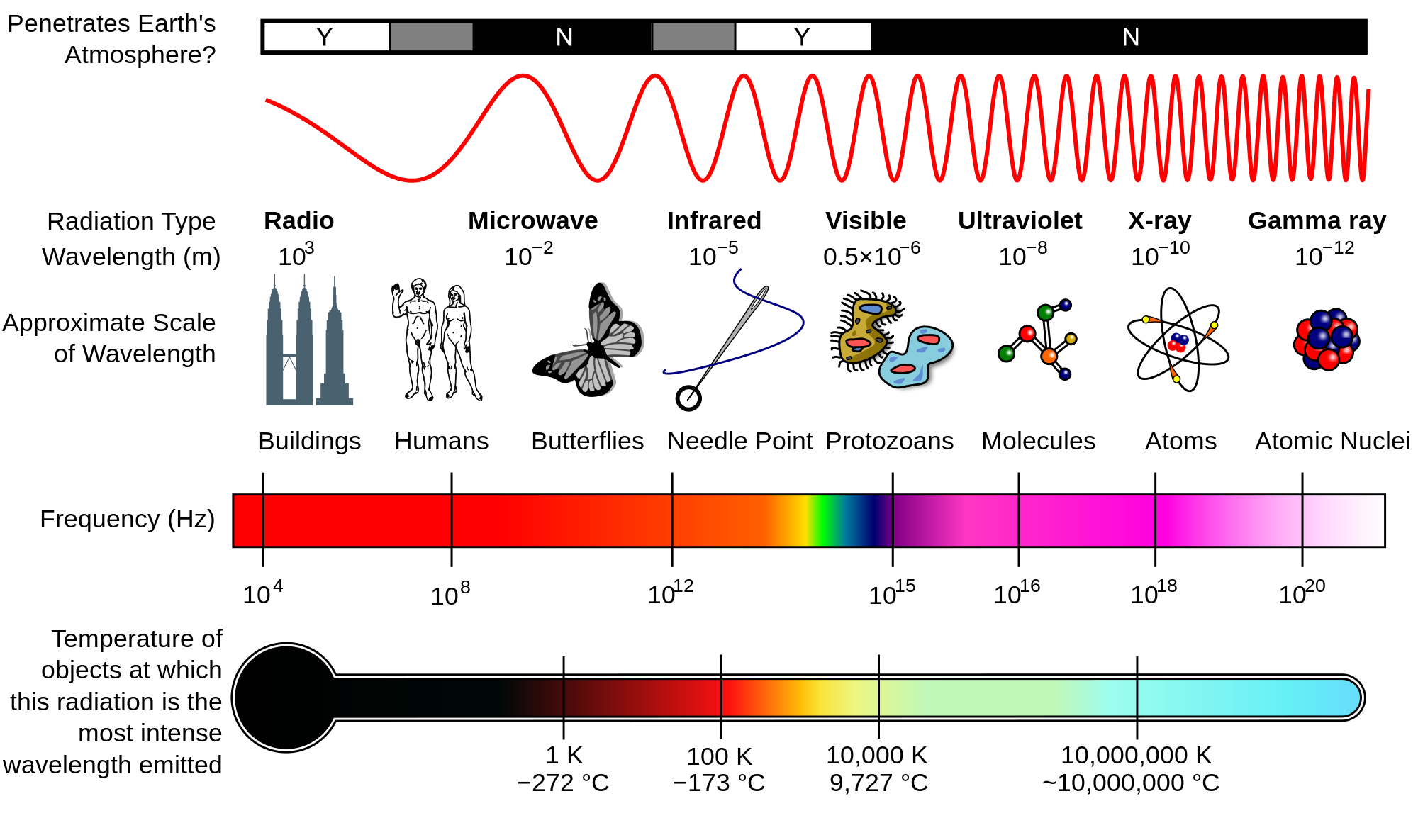
As you can see, this frequency places you in the Ultraviolet region of the EM Spectrum.

