The Balmer series corresponds to all electron transitions from a higher energy level to #n = 2#.
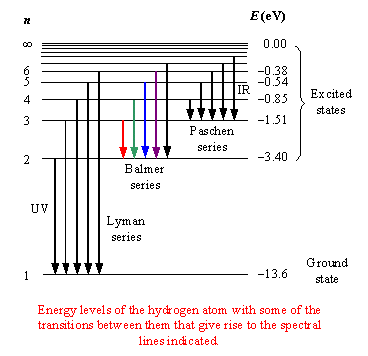
The wavelength is given by the Rydberg formula
#color(blue)(bar(ul(|color(white)(a/a) 1/λ = R(1/n_1^2 -1/n_2^2)color(white)(a/a)|)))" "#
where
#R =# the Rydberg constant and
#n_1# and #n_2# are the energy levels such that #n_2 > n_1#
Since #fλ = c#
we can re-write the equation as
#1/λ = f/c = R(1/n_1^2 -1/n_2^2)#
or
#f = cR(1/n_1^2 -1/n_2^2) = R^'(1/n_1^2 -1/n_2^2)#
where #R^'# is the Rydberg constant expressed in energy units (#3.290 × 10^15 color(white)(l)"Hz"#).
In this problem, #n_1 = 2#, and the frequency of the limiting line is reached
as #n → ∞#.
Thus,
#f = lim_(n → ∞)R^'(1/4 -1/n_2^2) = R^'(0.25 - 0) = 0.25R^'#
#= 0.25 × 3.290 × 10^15 color(white)(l)"Hz" = 8.225 × 10^14color(white)(l)"Hz"#


