What is the geometry around each of the three central atoms in the #CH_3COOH# molecule?
1 Answer
Feb 8, 2017
Here's what I get.
Explanation:
We must first draw the Lewis structure of acetic acid.
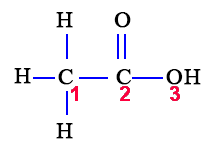
(Adapted from Chemistry@TutorVista.com)
Carbon
This atom has four atoms directly attached and no lone pairs.
Its electron geometry and its molecular geometry are both tetrahedral as in methane.

Carbon
This atom has three atoms directly attached and no lone pairs.
Its electron geometry and its molecular geometry are both trigonal planar.

Oxygen
This atom has two atoms directly attached and two lone pairs.
Its electron geometry is tetrahedral but its molecular geometry is bent as in water.
(From Meritnation)

