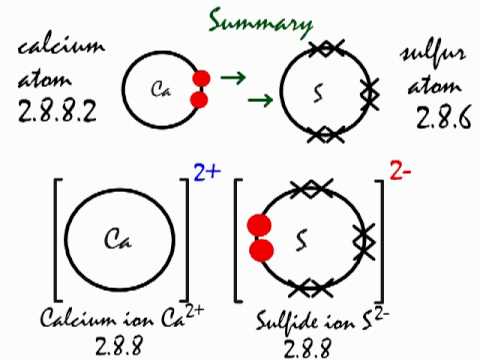
What is the ionic bond formation of calcium and sulfur?
1 Answer
Feb 2, 2014
In the ionic bond formation between calcium and sulfur, a calcium atom donates two valence electrons to a sulfur atom to form a
Explanation:
A Ca atom has two valence electrons. It can achieve a noble gas configuration by losing these two electrons.

(From Quora)
A sulfur atom has six valence electrons. It can achieve a noble gas configuration by gaining two electrons.

If Ca gives two electrons to S, both ions have a noble gas configuration.