What is the leaving group in the hydrolysis of tert-butyl bromide with water forming tert-butanol?
1 Answer
Sep 14, 2015
The
In a list of primary, secondary, and tertiary carbocations, the tertiary one is the most stable due to the most hyperconjugation stabilization between the methyl p orbitals and the empty p orbital in the center.
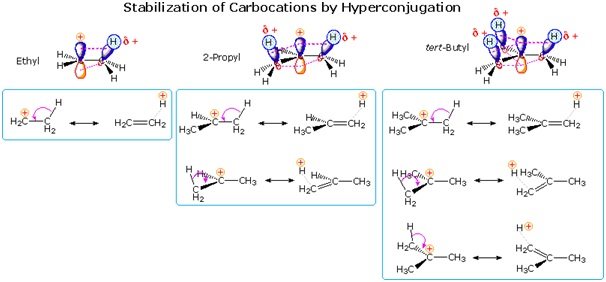
Therefore, it is reasonable to expect the bromide to leave when it coordinates with the water molecule in a first-order substitution (
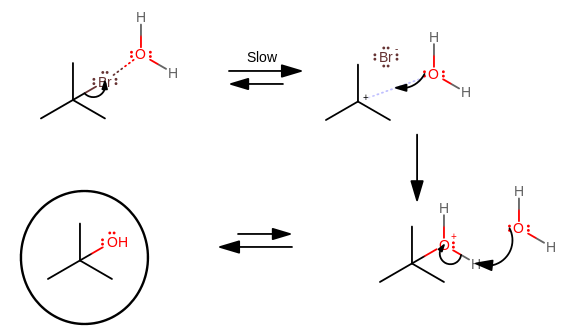
- The bromine "falls off" slowly due to a stable carbocation intermediate, but slowly because nothing else happens at the same time other than water coordinating with the bromine and slightly polarizing it
- Water becomes a nucleophile and adds onto the carbocation intermediate because the intermediate is now electrophilic enough
- Another water molecule (not
#Br^(-)# ) grabs a proton off of the protonated water substituent to finish the reaction since#HBr# is a much stronger acid than#H_3O^(+)# is

