What is the lewis dot structure of nitrate ion?
I have seen everyone draw the lewis dot structure of nitrate ion, putting 7 electrons around the oxygen atoms, while only 6 are supposed to be there. I see everyone doing the same thing. Please kindly explain.
I have seen everyone draw the lewis dot structure of nitrate ion, putting 7 electrons around the oxygen atoms, while only 6 are supposed to be there. I see everyone doing the same thing. Please kindly explain.
1 Answer
Well we got nitrate ion...and this gives
Explanation:
The nitrogen centre is quaternized, i.e. it formally bears a positive charge...because in the following resonance structures there are SIX ELECTRONS associated with it rather than the SEVEN electrons required for neutrality (we can count out the 4 valence electrons; there are 2 inner core electrons)....
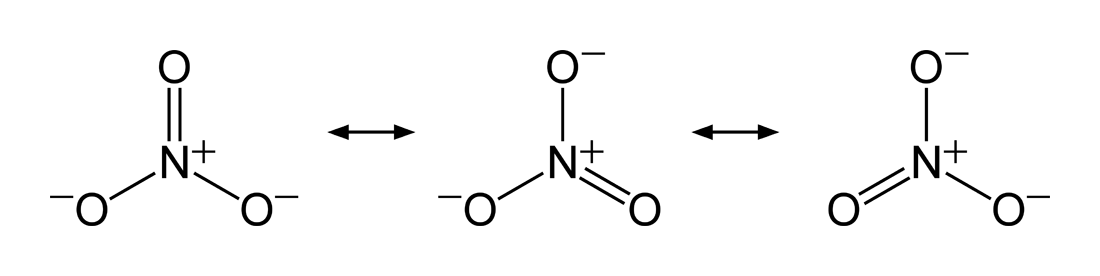
And thus for
For the parent nitric acid we would likewise write....

