What is the major factor that accounts for most of the difference in these two values of pressure (ideal gas law vs. van der Waals equation)?
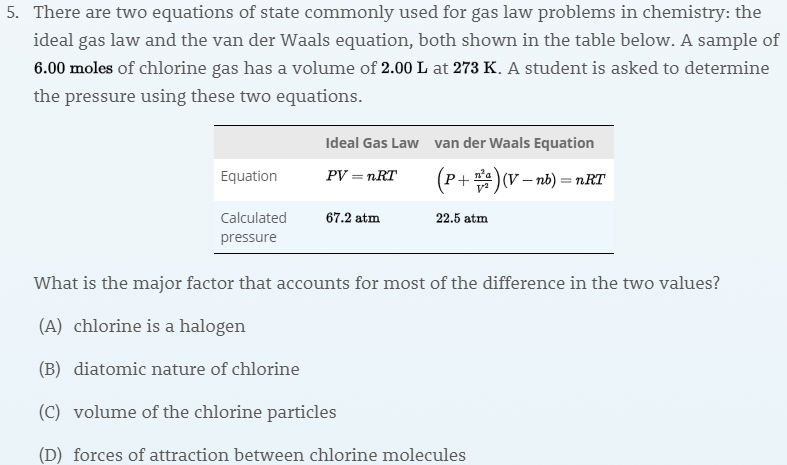
I'm not sure what the difference between B and D is? Why wouldn't they both affect it?

I'm not sure what the difference between B and D is? Why wouldn't they both affect it?
1 Answer
It's a matter of semantics. While
The above pressures say that since a lower pressure is actually needed to reach
#Z = (PV)/(nRT)#
is less than
#Z = (("22.5 atm")("2.00 L"))/(("6.00 mols")("0.082057 L"cdot"atm/mol"cdot"K")("273 K"))#
#~~ 0.3348 < 1#

