What is the major product formed in the following reaction?
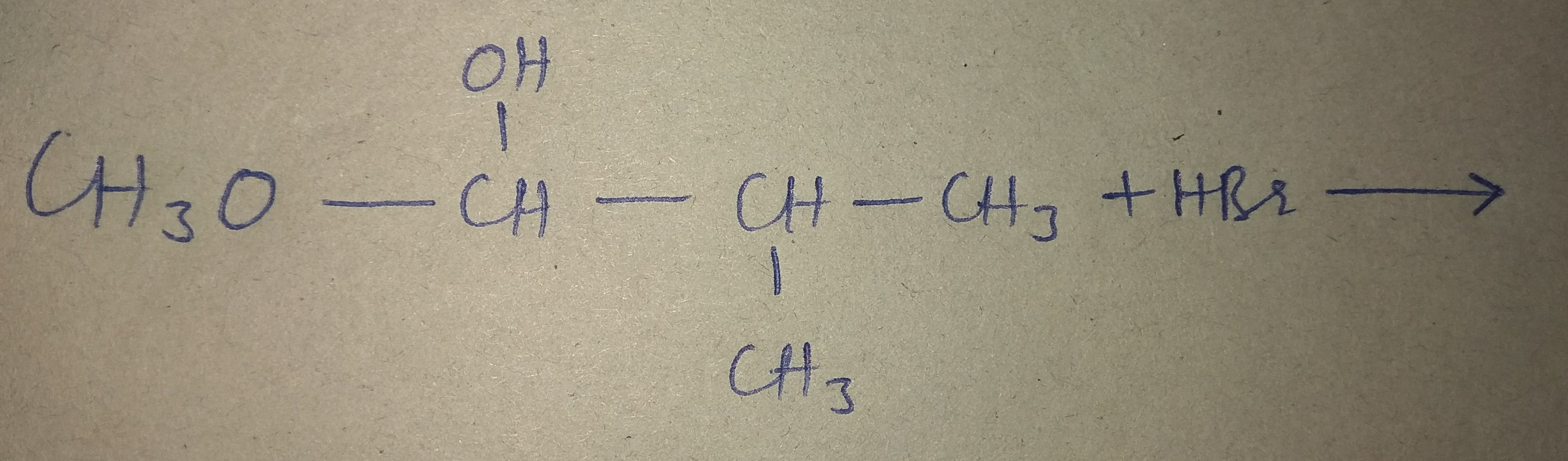
Answer is, there is Br in place of OH. Why there is no Hydride shift?
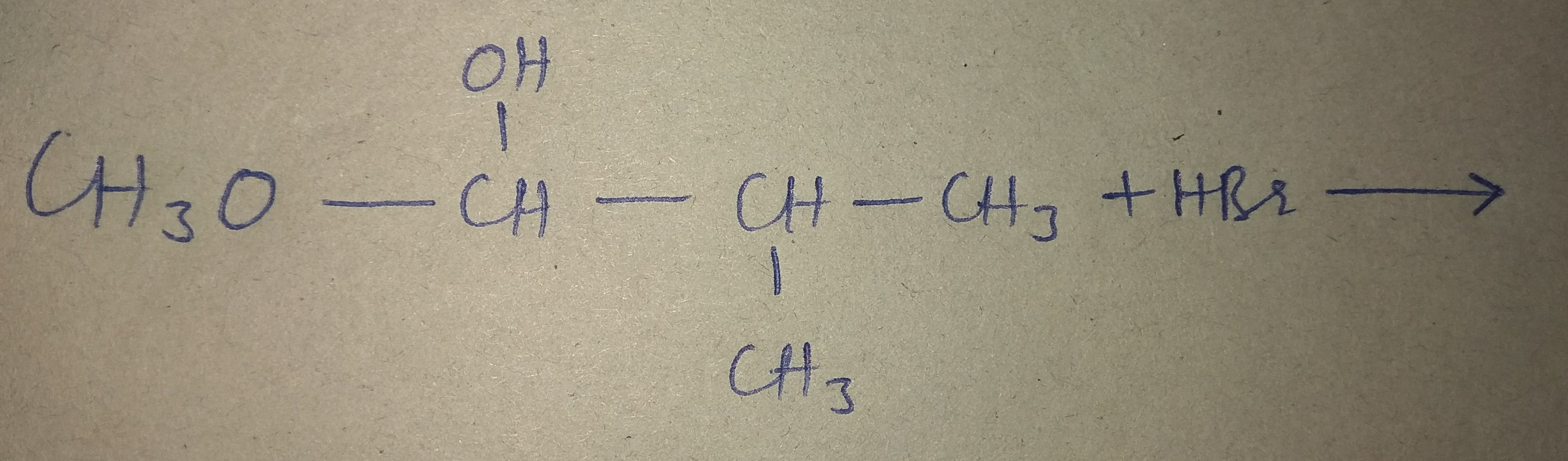
Answer is, there is Br in place of OH. Why there is no Hydride shift?
1 Answer
Mar 1, 2018
Consider the SN2 mechanism of your reaction,

Recall that SN2 is a concerted substitution reaction, so a carbocation intermediate is generally nonexistent.
Even if somehow there was a hydride shift, the reaction would yield nearly none of the tertiary alkyl product because there is far too much steric hindrance/electronic repulsion/molecular orbital misalignment for the nucleophile to attack effectively.
If this reaction underwent an SN1 mechanism, you would need to do a hydride shift, though.

