What is the mass of 5.99 mole of carbon monoxide?
1 Answer
Apr 29, 2016
263.56 g
Explanation:
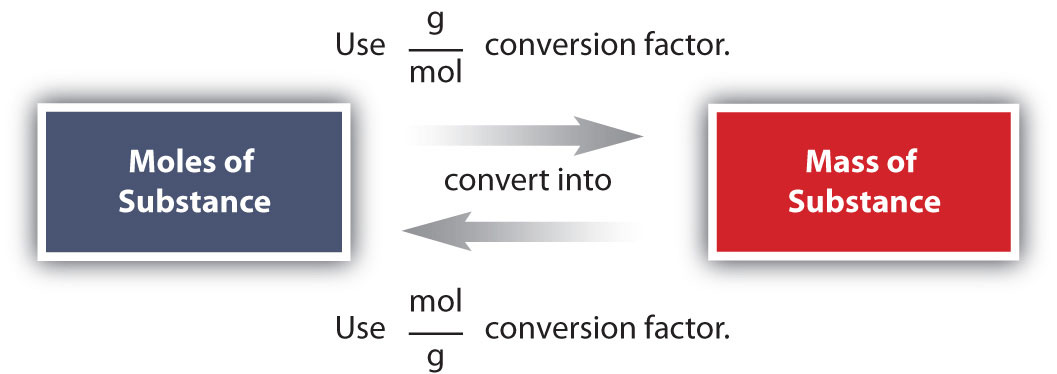
Mass of one mole of Carbon Monoxide is 44 g
1 mole of CO = 44 g
We can write two conversion factors using the above equation;
1 mole of CO / 44 g
number of moles : 5.99 mole
5.99 moles of CO x (44 g
263.56 g