What is the maximum number of grams of #"NH"_4"Cl"# that will dissolve in 200 grams of water at 70°C?
1 Answer
Explanation:
Your tool of choice here will be the solubility graph for ammonium chloride,
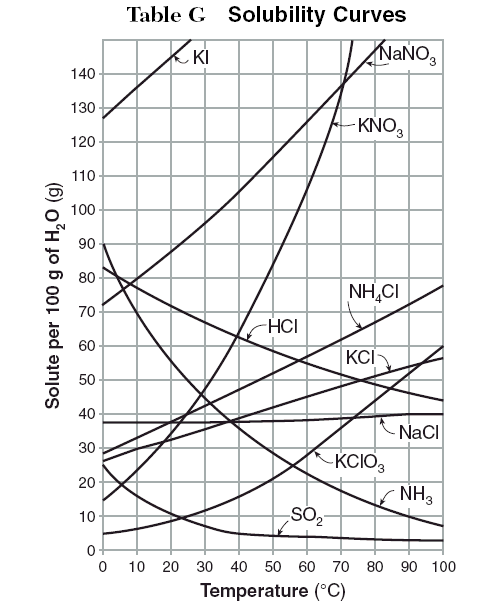
The solubility graph provides you with information on the solubility of a given salt at various temperatures. In this case, the graph shows you the solubility of ammonium chloride per
The actual curve represents the solubility of the salt, i.e. the maximum amount that can be dissolved in
At
After this point, any extra salt added would remain undissolved.
So, you know how much salt can be dissolved in
#200 color(red)(cancel(color(black)("g H"_2"O"))) * ("62 g NH"_4"Cl")/(100color(red)(cancel(color(black)("g H"_2"O")))) = "124 g NH"_4"Cl"#
I will leave the answer rounded to two sig figs, but keep in mind that you only have one sig fig for the mass of water
#"solubility at 70"""^@"C in 200 g H"_2"O" = color(green)(|bar(ul(color(white)(a/a)color(black)("120 g")color(white)(a/a)|)))#

