What is the molarity of a solution that contains 50.0 g of #Mg(NO_3)_2# per 225 mL of solution?
1 Answer
Jul 4, 2016
Explanation:
To find the molarity, the following equation must be used:
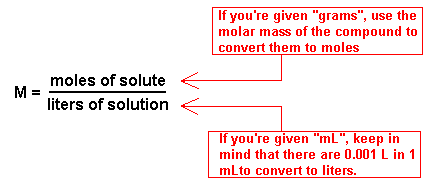
We are given the mass and volume of the solution, but both have bad units.
The mass of
Now we can convert 225 mL into L by multiplying 225 by 0.001 to obtain 0.225L.
All we do now is divide the number of moles by the volume to acquire the molarity:

