What is the most likely product to form when 4-methyl-1-pentene reacts with excess Br2? Please show the structural formula. What type of reaction is this?
1 Answer
Jun 21, 2018
The most likely product is 1,2-dibromo-4-methylpentane.
Explanation:
The reaction of an alkene with bromine gives a vicinal dibromide (a 1,2-dibromide).
Thus, the reaction of bromine with 4-methylpent-1-ene gives 1,2-dibromo-4-methylpentane.
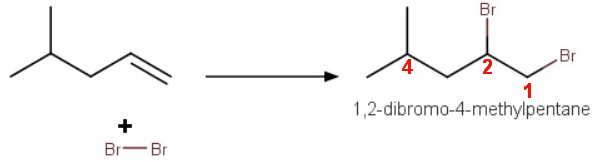
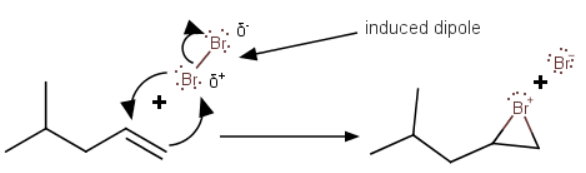
The mechanism
The electrons in the π bond of the approaching alkene induce a dipole in the highly polarizable
The π electrons attack the partially positive
The
In a second step, the
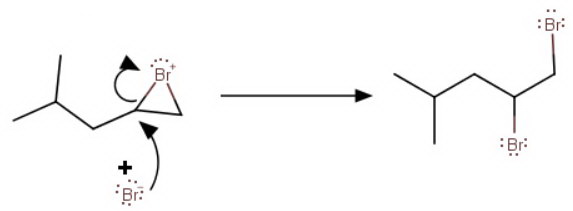
This reaction is an example of electrophilic addition to an alkene.

