What is the most stable conformation Of cis-1-4-dipropylcyclohexane?
1 Answer
Consider cis-1,4-dipropylcyclohexane,

The configuration here is evidently irrelevant, because this is an achiral molecule.
Moreover, consider the conformational analysis of cyclohexane,
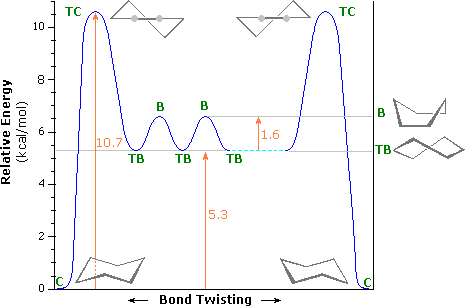
The most stable conformation is that which has the lowest energy. Generally, this means a chair conformation is the most stable conformation of cyclohexane.
This particular molecule has two fairly bulky substituents, so if both of them could be in equatorial positions on the chair, it would minimize steric hindrance with the axial hydrogens.
However, due to the locations of the substituents, only one can be equatorial in either flipped chair conformation. The A-value for propyls in the axial position is about
Hence, the likely answer for this question is,


