What is the most stable conformer for 3-methylpentane, viewed along the #C_2-C_3# bond using Sawhorse projections?
1 Answer
You can find the general procedure for drawing sawhorse projections at this location.
The condensed structural formula of 3-methylpentane is CH₃CH₂CH(CH₃)CH₂CH₃.
Its bond-line structure is
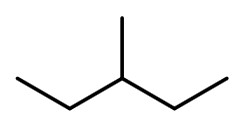
Step 1. Convert the bond-line structure to a wedge-dash structure at C-2 and C-3.
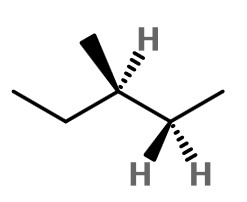
To convert the wedge-dash structure to a sawhorse projection, we must look at the molecule along the C2-C3 bond (from the lower right).
The three bonds nearest us form an inverted "Y".
We draw a sawhorse template with the C2-C3 bond going from lower left to upper right.
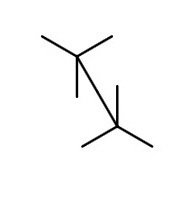
Next we add the atoms.
The groups on C-2 are CH₃, H, and H.
The groups on C-3 are CH₃CH₂, CH₃, and H.
On C-2, we put the bulky CH₃ group at the top of the inverted "Y", with the H atoms on the other two bonds.
On C-3, we put the bulky CH₃CH₂ group at the bottom of the "Y". The CH₃ goes on the left, and the H atom on the right.
The staggered sawhorse structure of 3-methylpentane is then
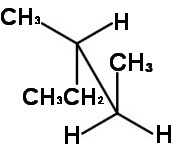
This is the most stable conformer because it puts the bulky CH₃ group on C-2 anti to the CH₃CH₂ group on C-3.

