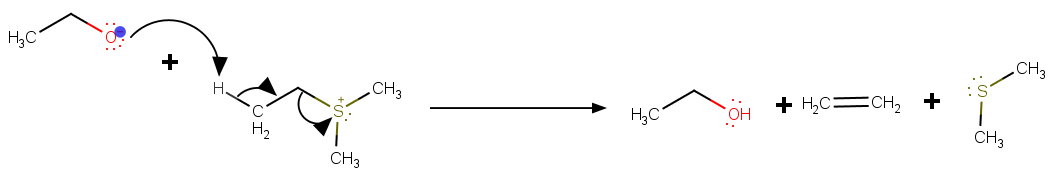
What is the name of the compound #CH_3CH_2S(CH_3)_2# and How does the following reaction happen?
#CH_3CH_2S(CH_3)_2 + C_2H_5ONa ->CH_3 CH_2OC_2H_5 + H_2C=CH_2#
Where, substitution = #88%# and elimination = #12%# . The reaction takes place in presence of ethanol.
EDIT: Added missing product: #"S"("CH"_3)_2#
- Truong-Son
Where, substitution =
EDIT: Added missing product:
- Truong-Son
1 Answer
Feb 22, 2018
Here's what I get.
Explanation:
The nucleophile/base is ethoxide ion,
The substrate is the ethyldimethylsulfonium ion,
The positive charge on the
The
The ethoxide acts as a nucleophile and attacks the α-carbon.
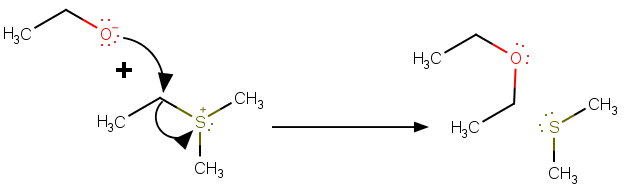
The
The ethoxide acts as a base and attacks the β hydrogen