What is the name of the product of the hydrogenation of 2-methylpropanal?
1 Answer
Dec 16, 2015
In general, the hydrogenation is supposed to reduce the compound by adding two hydrogens somewhere. The easiest place to add them is wherever the molecule is most electrophilic, so it would have to be across the carbonyl group.
The
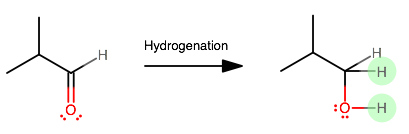
One actual example of hydrogenation (i.e. reduction) of an aldehyde is using
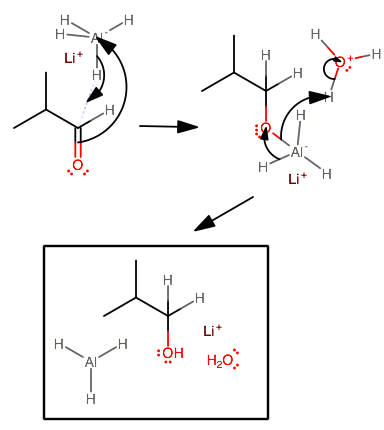
We see this weird behavior from hydrogen since

