What is the order of increasing acidic strength of the following compounds?
Is this question supposed to be solved on the basis of s character or will I also have to check for stability of conjugate base using resonance and inductive effect? I'm quite confused. Please tell me the correct order for both the questions and also how to do it.

Is this question supposed to be solved on the basis of s character or will I also have to check for stability of conjugate base using resonance and inductive effect? I'm quite confused. Please tell me the correct order for both the questions and also how to do it.
1 Answer
Apr 23, 2018
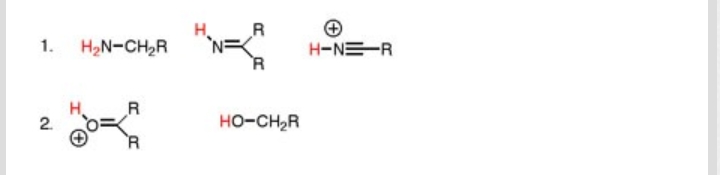
All of those factors matter!
The acidity of these protons depends on the stability of the conjugate base, having been deprotonated:
 puu.sh
puu.sh
Clearly
However, in increasing order
Lastly,

