What is the order of Ne, Sn, ge, S, and Rb in terms of ionization energy? How is this order determined?
1 Answer
Well ionization energies INCREASE across a Period......
And decrease down a Group.
Explanation:
Well ionization energies INCREASE across a Period.........as we look at the Table from LEFT to RIGHT; and they decrease down the Group. Two factors operate: (i) nuclear charge; and (ii) shielding by other electrons. Incomplete electronic shells shield the nuclear charge very ineffectively, with the result as we cross the Period from left to right nucleus-electron attraction raises the ionization energy. (This is also observed in the well known contraction of atomic radii across the Period from left to right).
And how is the order determined? How else but by experiment? Ionization energy is the energy required to produce one mole of positive ions, and one mole of electrons from one mole of gaseous atoms.....
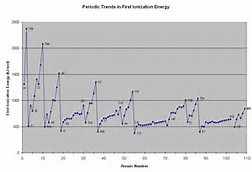
The graph clearly shows (or would if you could see the scale), the increase in ionization energies across the Period. When we move down a Period, ionization energies become less in magnitude, as indeed we would expect because the valence electron is farther removed from the nuclear charge.
And so the order of ionization energies (and you will have to find corroborating values) should DECREASE in the order:

