What is the pH of a #8.4x10^-6 M# #H^+# solution?
1 Answer
Aug 28, 2016
Explanation:
The pH can be obtained directly from the concentration using the formula below:
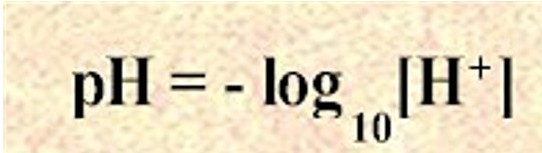
Take the -logarithm of the concentration of hydronium ions that are in the solution:

