What is the pH value when 450 mL 0.07 M NaOH is mixed with 550 mL 0.05 M CH3COOH? (pKs=4.75)
1 Answer
pH=11.6
Explanation:
The best way to tackle this problem is to determine which of the species is the analyte or the titrant. Usually, we could determine the titrant as a species that drives the reaction to become an irreversible reaction. This irreversible reaction would make titration a suitable way for analyzing concentrations of unknown species.
After we have determined the titrant the analyte would be the species that is being asked in the problem. So in this problem, the titrant is NaOH (Strong Base) and analyte is CH3COOH (weak acid also known as acetic acid or vinegar).
Now this would be a Strong Base and Weak Acid Titration process. We must create a table to properly account the number of moles reacting in this system.
First, convert the molarity into moles
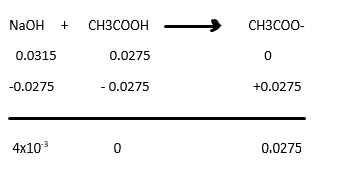
Now we could see that we both have 2 species contributing to the pH of the solution (
Now the pH is
pH=14.00-(-log(
pH= 11.6
∴ First, we find which is our titrant or analyte. This is a skill that would be harnessed if you read more and more about chemistry and solve more problems.
∴Second, we then change them into moles to carefully check which species remain that would act as a pH indicator of the system.
∴Third, get the concentration of NaOH because it is stronger base than of acetate ion where we could just disregard the effect of acetate ion to the pH of the system.
I do hope you learn something. If ever you have any questions or clarification don't hesitate to ask more questions. Thanks and God bless.
