What is the possible arrangement of the isomers of molecular formula C4H9Cl in order of decreasing rate of reaction with sodium iodide in acetone?
1 Answer
The order is butyl > isobutyl > sec-butyl > tert-butyl.
Explanation:
The reaction is an
#"I"^"-" + "R-Cl" → "I-R" + "Cl"^"-"#
Thus, a major factor affecting relative reaction rates is steric hindrance to attack by the nucleophile
Let's examine ball-and-stick and space-filling models of the isomeric chlorides.
In the space-filling models, I have marked the backside of the carbon bearing the leaving group with a yellow dot.
Butyl chloride
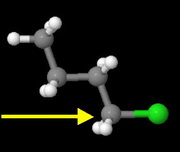
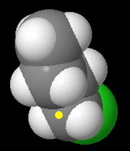
This is a primary alkyl halide.
It has the least steric hindrance to approach by the nucleophile, so it should have the fastest rate.
Isobutyl chloride
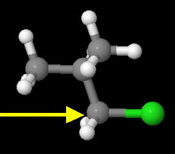

This is also a primary alkyl halide.
However, the branched isopropyl group provides more steric hindrance than the linear propyl group in butyl chloride.
This is the next fastest reacting chloride.
sec-Butyl chloride
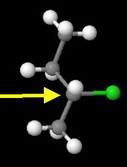
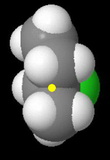
The methyl and ethyl groups severely hinder approach by the nucleophile. This is the third fastest reacting chloride.
tert-Butyl chloride
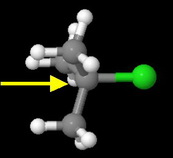
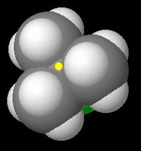
The carbon bearing the

