What is the product of adding KOH and Mg(OH)2 to these molecules?
a)p-hydroxybenzoic acid+KOH
b)p-hydroxybenzoic acid+Mg(OH)2
a)p-hydroxybenzoic acid+KOH
b)p-hydroxybenzoic acid+Mg(OH)2
1 Answer
Aug 3, 2017
Well, my instinct is... that the only thing that occurs is deprotonation and charge stabilization.
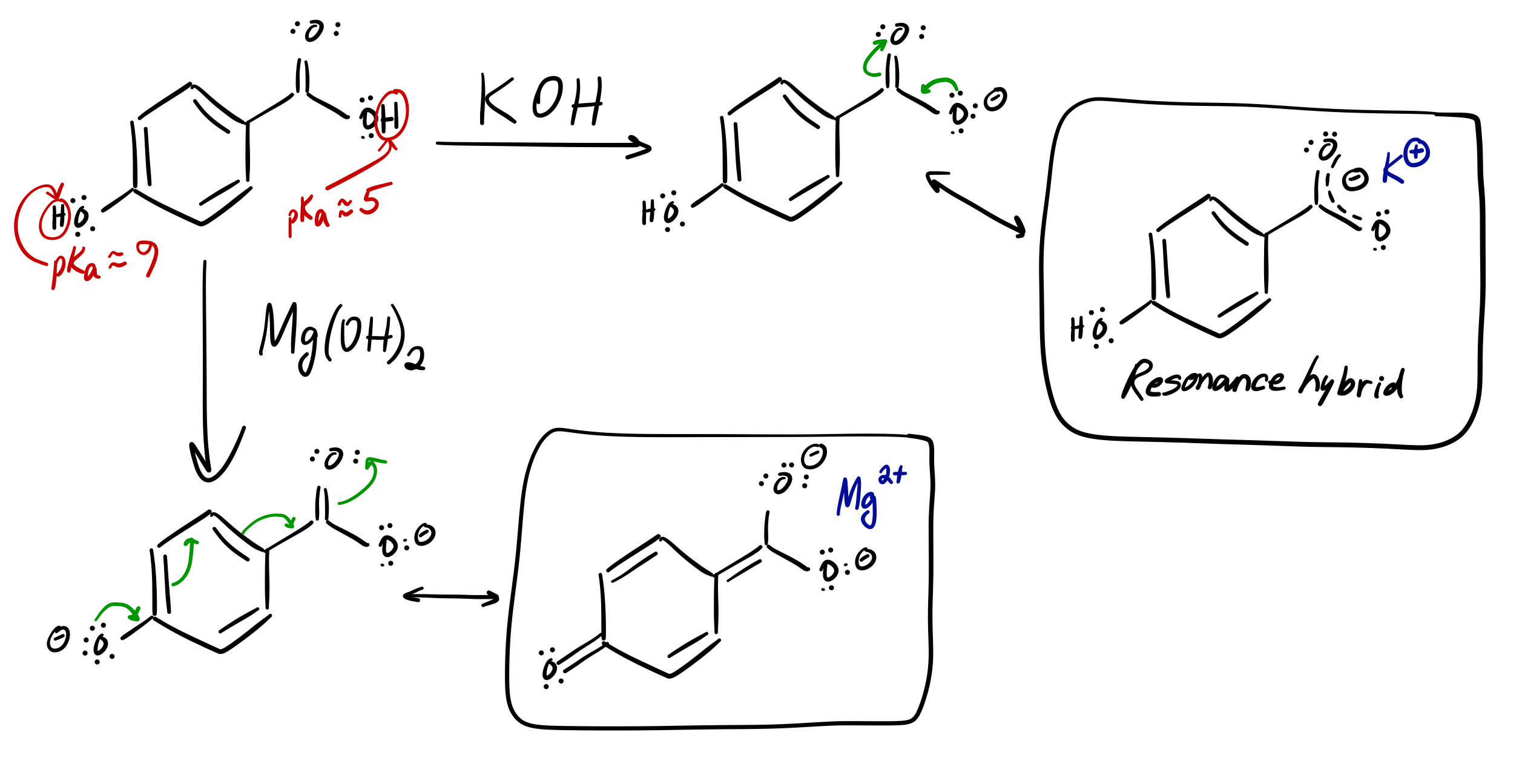
The carboxylic acid proton should be the first one deprotonated in preference to the hydroxyl proton, given that its

