Why is the product of HBr with (R)-3-methyl-3-hexanol going to be 3-brom-3-methylhexane?
Reaction of HBr with (R)-3-methyl-3-hexanol gives racemic mixture 3-brom-3-methylhexane. What type of reaction is this, write the mechanism and explain why do we get this type of product?
Reaction of HBr with (R)-3-methyl-3-hexanol gives racemic mixture 3-brom-3-methylhexane. What type of reaction is this, write the mechanism and explain why do we get this type of product?
1 Answer
A racemic mixture (
(R)-3-methyl-3-hexanol is a tertiary alcohol, which is bulky. Thus, at ordinary temperatures, it tends to undergo
It also follows that formation of a carbocation intermediate is easier (because more hyperconjugation stabilizes the central cationic carbon).
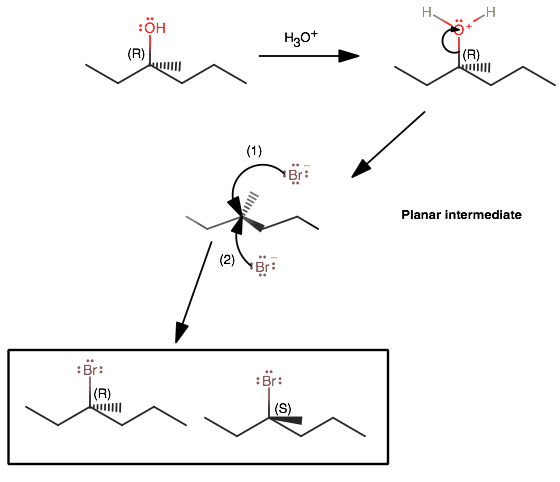
The loss of the water molecule (the leaving group) is the slow step in the mechanism (with NO participation of
This is why the mechanism is first order with respect to the substrate, i.e.
Since the nucleophilic attack of the intermediate is fast, the product that forms is almost entirely dependent on the planarity of the intermediate.
In other words, either face could be attacked, and the umbrella flip is equally as likely to go either way (either retaining or inverting the stereochemistry), thus giving a 50/50 mixture.
This is contrary to the
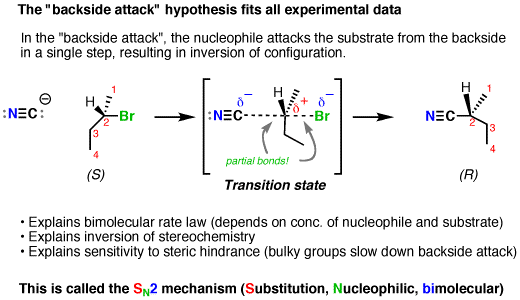
Since no carbocation intermediate forms, only a one-directional back-side attack can occur, which umbrella-flips to only invert the stereochemistry in a second-order reaction with respect to the substrate.

